Reprogramming of energy metabolism is one of the hallmarks of cancer, since tumour cells need to fuel their high proliferation rates.1 Warburg et al.2 first observed that cancer cells largely limit their method of energy production to glycolysis only. In order to compensate for lower ATP yields, cancer cells often upregulate expression of glucose transporters, typically GLUT1, which substantially increases extracellular glucose import into the cytoplasm.3
In normal physiology, insulin-responsive cells rapidly increase their glucose uptake 10–20-fold by translocating glucose transporter vesicles (specifically GLUT4) from intracellular storage compartments to the plasma membrane. In adipocytes, insulin activates the protein kinase AKT to phosphorylate the Rab GTPase activating protein, TBC1D4. This protein normally maintains Rab GTPases in their inactive state so that storage vesicles containing the glucose transporter remain cytoplasmic. Upon phosphorylation, TBC1D4 becomes inactive, allowing the translocation of GLUT vesicles so that more transporters are inserted into the plasma membrane.4 In muscle cells, the highly homologous Rab-GTPase activating protein TBC1D1 is predominantly expressed but also becomes phosphorylated by AKT in response to insulin.
Previous work from our laboratory described that the protein kinase WNK1 also participates in the regulation of TBC1D4. WNK1 forms a protein complex with TBC1D4 in human embryonic kidney (HEK293) cells and phosphorylates it in vitro. Phosphorylation by WNK1 was found to increase the binding of TBC1D4 to regulatory 14-3-3 proteins and to reduce its interaction with the exocytic small GTPase, Rab8A. This novel pathway regulates the cell surface expression of GLUT1 (Figure 1).5
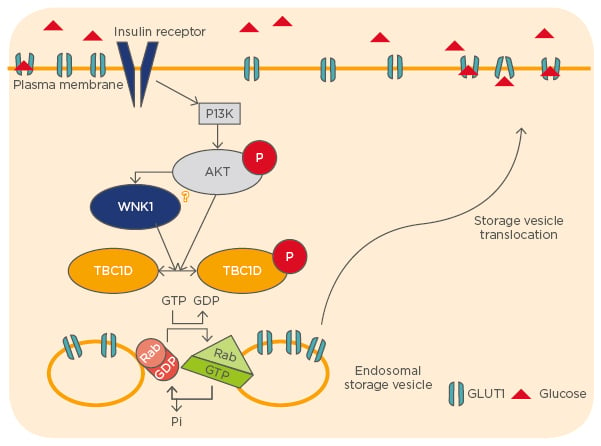
Figure 1: The regulation of cell surface expression of GLUT1.
GLUT1: glucose transporter 1; PI3K: phosphoinositide 3-kinase.
The subfamily of WNK protein kinases belongs to the superfamily of 518 human protein kinase genes.6,7 WNK1 and WNK4 were shown to regulate the cell surface expression and activity of a variety of renal ion channels.7 The finding that WNK1 regulated GLUT1 showed, for the first time, that it is also involved in the cell surface expression of other plasma membrane transport proteins.5 Besides the catalytic domain, WNK1 contains coiled-coil domains, which, together with the large size of the protein, suggests that WNK1 also has scaffolding functions and may be involved in multiple cellular signalling processes.6-8 Interestingly, AKT can phosphorylate WNK1 at residue Thr60.9
Our goal was to characterise the role of protein kinase WNK1 in the phosphorylation network that regulates cellular glucose uptake in cancer cells. WNK1 expression was depleted by small interfering RNA in various colorectal cancer cell lines and key cell cycle proteins were analysed by a western blot in the presence of different glucose concentrations. WNK1-depleted cells showed higher apoptotic and cell cycle arrest phenotypes when cultured in low glucose medium.
In order to determine how WNK1 regulates GLUT1 translocation, key phosphorylation events were identified by mass spectrometry analysis, which revealed unique serine residues specifically phosphorylated by WNK1 in both TBC1D4 and the functionally related TBC1D1. The respective phosphomimetic mutants are currently being tested for their ability to modulate GLUT1 translocation. Together, these studies will contribute to an increased knowledge of the signalling pathways involved in the control of glucose uptake in cancer cells and will potentially allow the identification of novel therapeutic targets.








