At the European Society for Medical Oncology (ESMO) 2018 Congress, data from the ongoing Phase I clinical trial of the anti-programmed death (PD)-1 monoclonal antibody TSR-042 were presented. Blocking PD-1 has been shown to increase antitumour immune responses and overall survival of patients with multiple tumour types.1 TSR-042 is an investigational humanised monoclonal antibody that targets PD-1, effectively blocking interaction with its ligands PD-L1 and PD-L2. TSR-042 is being evaluated in patients with advanced solid tumours in the ongoing Phase I GARNET trial.2 Safety and efficacy data from the microsatellite instability-high (MSI-H) endometrial cancer (EC) cohort, as well as pharmacokinetics (PK) and receptor occupancy (RO) at the recommended Phase II dose (RP2D), were presented.
Patients with previously treated MSI-H EC were evaluated and received TSR-042 500 mg every 3 weeks for the first four cycles and 1,000 mg every 6 weeks thereafter. Antitumour activity was assessed by immune-related Response Evaluation Criteria in Solid Tumors (irRECIST). Serum and peripheral blood mononuclear cells were collected for PK and RO measurements, respectively.
At the time of the analysis, 35 patients with MSI-H EC were treated with TSR-042; the median age was 63 years, 37% of patients had International Federation of Gynecology and Obstetrics (FIGO) Stage I cancer at diagnosis, and 34% had FIGO Stage III. The median number of prior regimens was two (range: 1–4). Of the 25 evaluable patients (≥12 weeks of follow-up in the study), a response was seen in 13 patients and the overall response rate was 52% (95% confidence interval: 31.3–72.2). One patient had a complete response and partial responses (PR) occurred in 12 patients (48%), including 1 patient with unconfirmed PR who is ongoing in the study. In addition, 3 patients (12%) had stable disease, 7 patients (28%) had progressive disease, and 2 patients did not have an evaluable tumour assessment (Figure 1). The median duration of response was not reached and 12 of the 13 responders (92%) are ongoing in the study. Three patients with a PR have been receiving TSR-042 for >60 weeks.
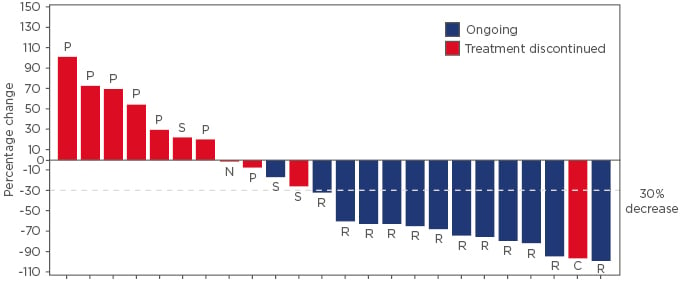
Figure 1: Best percentage change in total tumour burden from baseline based on immune-related Response Evaluation Criteria in Solid Tumors (irRECIST) in patients with recurrent or advanced microsatellite instability-high endometrial cancer.*
*One patient out of the 25 evaluable patients did not have a post-baseline tumour assessment and was not included in this waterfall plot.
C: immune-related complete response; N: not evaluable; P: immune-related progressive disease; R: immune-related partial response; S: immune-related stable disease.
Of the 35 patients, 23 (66%) had ≥1 treatment-related treatment-emergent adverse event. Grade ≥3 treatment-related treatment-emergent adverse events were reported in 4 patients (11%) and were noted as leukopenia, neutropenia, anaemia, alanine aminotransferase increased, and aspartate aminotransferase increased. No treatment-related death or treatment discontinuation was reported. TSR-042 exhibited linear and dose-proportional PK, and maintained serum concentrations at least 8-fold higher than required for full RO throughout the course of treatment.
These preliminary efficacy data indicate robust clinical activity of TSR-042 in patients with previously treated recurrent or advanced MSI-H EC and a safety profile consistent with approved PD-1 inhibitors. Safety and efficacy data for TSR-042 at the RP2D support the unique and convenient dosing schedule of 500 mg every 3 weeks for the first four cycles and 1,000 mg every 6 weeks thereafter. PK was consistent across patients and full RO was achieved at RP2D.








