BACKGROUND
Inducible T cell costimulator (ICOS), a member of the CD28/B7 receptor superfamily, is expressed on T cells after T cell receptor engagement with cognate antigen.1 ICOS provides a costimulatory signal augmenting T cell expansion, function, and survival, and is involved in B cell function.2-4 GSK335609 is a humanised IgG4 antibody engineered to reduce Fc-mediated depleting effects yet retain cross-linking for potent agonist activity.5 Engagement of ICOS to stimulate agonist function is hypothesised to translate into an optimal therapeutic effect.4,6 GSK3359609’s unique mechanism offers an opportunity to investigate the antitumour potential of targeting a T cell costimulator alone and in combination.
METHODS
INDUCE-1 is a dose escalation (DE) and ongoing expansion phase study of GSK3359609 alone (Part 1) and in combination with pembrolizumab (Part 2).7 The modified toxicity probability interval informed DE decisions with three or more patients enrolled per dose level (DL). GSK3359609 was administered as an intravenous infusion every 3 weeks (Q3W) with or without 200 mg pembrolizumab Q3W; treatment continued up to 2 years or until progression or unacceptable toxicity. To be included in the study, patients needed to have metastatic or relapsed invasive malignancy, measurable disease, received five or fewer lines of prior therapy in the advanced setting, adequate organ function, and no active autoimmune disease requiring treatment; the PK/PD cohorts required pre-treatment and Day 43 on-treatment tumour biopsies. The primary objective was to determine safety, tolerability, and maximum tolerated (MTD) GSK3359609 dose.
RESULTS
In the DE phase and the PK/PD cohort, 98 patients enrolled. In Part 1, 22 patients enrolled in the DE cohort and 40 patients enrolled in the PK/PD cohort. In Part 2, 36 patients enrolled in the DE cohort. Most patients had microsatellite stable colorectal carcinoma (26%) and ≥2 baseline target lesions (57%); 37% had received ≥3 prior lines of therapy in the advanced setting and 31% prior anti-programmed cell death protein 1/ligand-1 therapy. In Part 1 (n=62), 22 patients (35%) had at least one treatment-related adverse event (TR-AE). The most frequent TR-AE (≥3 patients) were fatigue (15%), aspartate transaminase (AST) elevations (5%), and diarrhoea (3%); AST elevations were the most frequent Grade 3 or 4 TR-AE (n=2 [3%]). In Part 2, 15 patients (42%) had at least one TR-AE; the most frequent TR-AE were AST elevations (8%) and pyrexia (8%); no Grade 3 or 4 TR-AE occurred in >1 patient. One DLT occurred in DE: Grade 3 pneumonitis in a Part 2 patient treated at the top GSK3359609 DL of 3 mg/kg, which led to discontinuation of both drugs. In the PK/PD cohort, liver enzyme increases in one patient (GSK3359609 3 mg/kg) were DLT and the only TR-AE leading to treatment discontinuation. Disease progression was the primary reason for treatment discontinuation (92%). Approximate dose proportional increases in systemic GSK3359609 concentrations over 0.01–3.00 mg/kg DL were observed. At DL ≥0.3 mg/kg, ICOS receptor occupancy was ≥75% across the dosing interval. On-target PD effects in tumour infiltrating lymphocytes and clinical activity were observed in Part 1 and 2, including in anti-programmed cell death protein 1/ligand-1 therapy experienced patients (Figure 1).
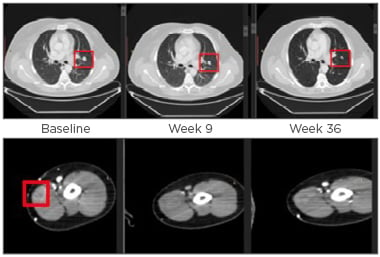
Figure 1: Axial CT images of left upper lobe lung lesion and subcutaneous left bicep lesion.
Fifty-three-year-old male diagnosed with Stage IIIc, BRAF/cKIT mutation-negative nodular melanoma; previous regimens included treatment with the combination of ipilimumab and nivolumab for approximately 2 months (BoR was SD); nivolumab alone for approximately 1 year (BoR was SD). At baseline, total disease burden was five target lesions (sum of diameters was 225 mm) and multiple non-target lesions. In this study, the patient received GSK3359609 monotherapy in three 0.1 mg/kg doses until Week 48. After Week 48, the dose was 1 mg/kg; the BoR was PR.
BoR: best overall response; PR: partial response; SD: stable disease.
CONCLUSION
GSK3359609 alone and in combination with pembrolizumab was well-tolerated across the 0.001–3.000 mg/kg dose range. MTD was not reached; the maximum administered dose was 3 mg/kg. A range of doses (≥0.1–1.0 mg/kg) showed biological and clinical activity. These doses are under investigation in expansion cohorts to establish the GSK3359609 dose and assess clinical activity across different patient groups. Preliminary biological and clinical data support the mechanism of action of a non-depleting ICOS agonist as a clinical target.








