BACKGROUND AND AIMS
High incidence of lung cancer in Serbia is derived from high smoking rates, the lack of a nationwide screening programme, and the presence of environmental risk factors.1 By histology, adenocarcinoma accounts for approximately 50% of all lung cancer cases, with approximately 60% of new diagnoses already in advanced disease stages, where targeted therapies and immunotherapy are indicated. In Serbia, epidermal growth factor receptor (EGFR) mutation testing is still performed on liquid biopsy of patients with lung cancer only by quantitative PCR.2,3 The aim of this study was to evaluate the efficacy of this approach in pleural effusions of patients with non-small cell lung cancer (NSCLC) at diagnosis and after progression on EGFR tyrosine kinase inhibitors (EGFR-TKI) in Serbia.
MATERIALS AND METHODS
Mutation testing was performed on blood and pleural effusions of patients with advanced lung adenocarcinoma (Stage IIIB or IV; Eastern Cooperative Oncology Group [ECOG] Performance Status 0, 1, or 2) using the Cobas® EGFR Mutation Test (Roche, Basel, Switzerland), which detects 42 mutations in exons 18, 19, 20, and 21 of the EGFR gene with sensitivity of 5%.
RESULTS
In the period from 2016–2021, 124 patients were tested at baseline (53% males; 47% females; age range 37–83 years; median 61), and 104 after progression on first line EGFR-TKIs (41% males; 59% females; age range: 34–81 years; median age: 60). At diagnosis, nine mutated samples were detected (7.3%) with a turnaround time of 2 working days, and a 99.2% testing success rate. At progression, an accordance rate of only 67% with the initial driver mutation was observed from blood. The T790M mutation was detected in 34 patients (49% of the mutated samples; 33% of the total), which rendered them eligible for third generation EGFR-TKIs. In the same period, only six samples (4.8%) of pleural effusions were sent to our laboratory along with blood, all from patients at progression. The testing was performed with a turnaround time of 2 working days, a 100% testing success rate, and an accordance rate of 100% with the initial driver mutation. In 50% of concurrently sent samples, the T790M mutation was detected in the pleural effusion, while the corresponding blood sample showed only the presence of the driver mutation (Figure 1).
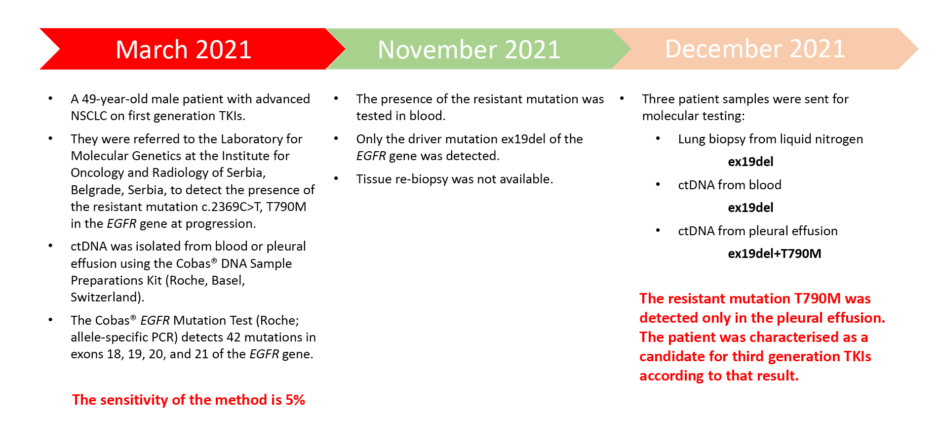
Figure 1: A Presentation of a clinical case from the Laboratory for Molecular Genetics, Institute for Oncology and Radiology of Serbia, Belgrade, Serbia.
ctDNA: circulating tumour DNA; NSCLC: non-small cell lung cancer; TKI: tyrosine kinase inhibitors.
A 49-year-old male patient with advanced NSCLC on first generation TKIs was referred to the Laboratory for Molecular Genetics at the Institute for Oncology and Radiology of Serbia, Belgrade, Serbia to detect the presence of the resistant mutation T790M in the EGFR gene at progression. The presence of the resistant mutation was first tested in blood, but only the driver mutation ex19del was detected. One month later, three additional patient samples were sent for molecular testing with following results: lung biopsy from liquid nitrogen (ex19del), circulating tumour DNA from blood (ex19del), and circulating tumour DNA from pleural effusion (ex19del+T790M). The resistant mutation T790M was detected only in the pleural effusion. The patient was characterised as a candidate for third generation TKIs according to that result.
CONCLUSION
EGFR mutation testing from pleural effusion has proven valuable as an alternative liquid biopsy sample to blood, for detecting the T790M acquired resistance mutation in patients with advanced NSCLC who progressed on first-line EGFR-TKIs in Serbia. Pleural effusion samples should be considered as a valuable source of genetic material and might be proposed by future guidelines for concurrent EGFR mutation testing alongside blood whenever available.4,5








