BACKGROUND AND AIMS
Most patients with glioblastoma will develop a recurrence despite initial aggressive therapy with maximum surgical resection and postoperative chemoradiation.1
A re-excision is often not feasible, but palliative chemotherapy can be considered depending on fitness, performance status, and other patient factors. In the absence of Level 1 evidence, different chemotherapy regimens have been used in this setting. The aim of this study was to evaluate an approach that included rechallenge temozolomide (TMZ) for patients with disease-free survival greater than 6 months after initial therapy, and procarbazine/lomustine/vincristine (PCV) for those exhibiting shorter responses to initial treatment.
MATERIALS AND METHODS
The authors retrospectively collected and analysed the data of patients with glioblastoma treated with systemic therapy for recurrent disease during 2009–2019. After the initial diagnosis, they all had a surgical resection and received treatment on the Stupp protocol.2 The Response Assessment in Neuro-Oncology (RANO) criteria3 were used to assess response to palliative chemotherapy after recurrence. Patient demographics and disease/treatment characteristics were described and survival outcomes were estimated using the Kaplan–Meier method. Haematological toxicities were recorded and chemotherapy-related admission rates were used as surrogate for other toxicities.
RESULTS
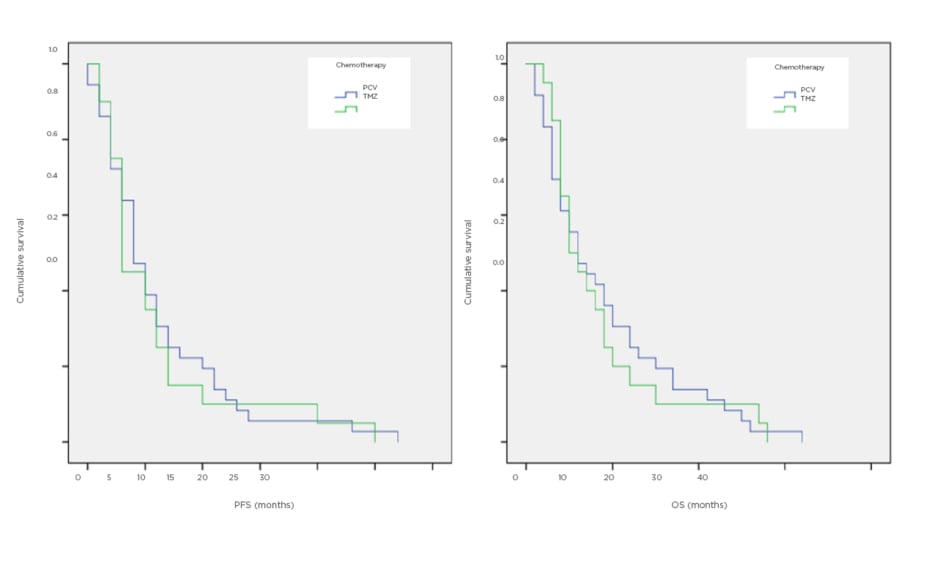
Fifty-six patients were identified, of whom 20 received rechallenge TMZ and 36 received PCV. Measured from the start of palliative chemotherapy, the median progression-free survival was 3 months for TMZ and 4 months for PCV, while median overall survival was 5.5 months for TMZ and 6 months for PCV (Figure 1).
Figure 1: Survival curves for progression-free and overall survival, comparing temozolomide and procarbazine/lomustine/vincristine.
OS: overall survival; PCV: procarbazine/lomustine/vincristine; PFS: progression-free survival; TMZ: temozolomide.
Both regimens were reasonably well tolerated. Grade 3–4 haematological toxicity was 10% and Grade 1–2 was 25% with TMZ. Corresponding figures for PCV were 13.9% and 30%. Only one patient was admitted into hospital for pneumonia (who had received PCV).
CONCLUSION
Rechallenging patients with recurrent glioblastoma with TMZ after a durable initial response to surgery and chemoradiotherapy, and offering PCV to those with a worse response to primary therapy, is a reasonable, pragmatic approach adopted by many UK cancer centres. Of interest, patients treated with rechallenge TMZ had similar progression-free and overall survival as their PCV counterparts, despite belonging in a theoretically better prognosis group (in view of a better response to primary treatment). This toxicity data suggests this treatment protocol is safe.