Authors: John DeLuca,1,2 Massimo Filippi,3 Ralf Gold,4 Krzysztof W. Selmaj,5,6 Robert Zivadinov7
1. Kessler Foundation, East Hanover, New Jersey, USA
2. Departments of Physical Medicine and Rehabilitation, and Neurology, Rutgers-New Jersey Medical School, Newark, USA
3. Neuroimaging Research Unit, Division of Neuroscience, Neurology Unit, Neurorehabilitation Unit, and Neurophysiology Service, IRCCS San Raffaele Scientific Institute, and Vita-Salute San Raffaele University, Milan, Italy
4. Neurologische Universitätsklinik, St. Josef Hospital, Bochum, Germany
5. Centre for Neurology, Łódź, Poland
6. Collegium Medicum, University of Warmia and Mazury, Olsztyn, Poland
7. Buffalo Neuroimaging Analysis Centre, Department of Neurology, Jacobs School of Medicine and Biomedical Sciences, University at Buffalo, The State University of New York, USA
Disclosure: DeLuca has received consultancy fees from Biogen Idec, Bristol Myers Squibb (BMS), Janssen Pharmaceuticals, and Novartis; speaker fees for Consortium of MS Centres; and grant funding from Biogen Idec, Canadian MS Society, Consortium of MS Centers, EMD Serono, and National MS Society. Filippi is the editor-in-chief of the Journal of Neurology and associate editor of Human Brain Mapping, Neurological Sciences, and Radiology; has received compensation for consultancy services from Alexion, Almirall, Biogen, Merck, Novartis, Roche, and Sanofi; speaker fees from Bayer, Biogen, Celgene, Chiesi Italia SpA, Eli Lilly, Genzyme, Janssen Pharmaceuticals, Merck Serono, Neopharmed Gentili, Novartis, Novo Nordisk, Roche, Sanofi, Takeda, and Teva; has participated in advisory boards for Alexion, Biogen, BMS, Merck, Novartis, Roche, Sanofi, Sanofi-Aventis, Sanofi-Genzyme, and Takeda; has provided scientific direction for educational events for Biogen, BMS, Celgene, Lilly, Merck, Novartis, Roche, and Sanofi-Genzyme; and receives research support from Biogen Idec, Fondazione Italiana Sclerosi Multipla, Italian Ministry of Health, Merck Serono, Novartis, and Roche. Gold has received research support and speaker fees from Abbott, Bayer Schering, Biogen, BMS, Chugai, Eisai, Janssen, Merck Serono, Novartis, Nikkiso Pharma, Roche, Sanofi-Genzyme, and Teva; consultancy fees from ZLB Behring, Baxter, and Talecris; and personal stock options in Bayer, Merck, Novartis, Pfizer, and Roche. Selmaj is a consultant for Biogen, Celgene, Genzyme, Merck, Novartis, Ono Pharma, Roche, Synthon, and Teva. Zivadinov has received speaker/consultancy fees from BMS, EMD Serono, Janssen Pharmaceuticals, Mapi Pharma, Novartis, Sanofi, and 415 Capital, and financial support for research activities from BMS, CorEvitas, EMD Serono, Mapi Pharma, Novartis, Protembis, and V-Wave Medical.
Acknowledgements: Writing assistance was provided by Nicola Humphry, Nottingham, UK.
Support: The publication of this article was supported by Bristol Myers Squibb.
Disclaimer: The opinions expressed in this article belong solely to presenters.
Keywords: Brain volume, cognitive functioning, long-term, relapse, relapsing multiple sclerosis (RMS), rebound effect, ozanimod, safety.
Citation: Neurol AMJ. 2024;1[1]:24-33. https://doi.org/10.33590/neurolamj/ZRZL5766.
Meeting Summary
Ozanimod is an approved treatment for relapsing multiple sclerosis (RMS) that has been shown to reduce relapses, new brain lesions, and brain volume loss relative to intramuscular interferon (IFN) β-1a.This article summarizes the latest data, and several new analyses, of clinical trials of ozanimod in RMS, which were presented at the 9th Joint European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS)-ACTRIMS Meeting in 2023, and the Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) Forum 2024.
ENLIGHTEN is a prospective, open-label study of ozanimod in patients with early RMS (≤5 years after diagnosis of multiple sclerosis [MS]) who have received ≤1 MS disease-modifying therapy (DMT). In an ad hoc interim analysis, conducted after 1 year, cognitive processing speed improved or remained stable in the majority of patients. This suggests that ozanimod may prevent cognitive decline during the first year of use. In addition, decline in whole brain volume (WBV), which is often accelerated in patients with MS, was minimal, indicating that brain volume was preserved during the first year of ozanimod treatment in patients with early RMS.
Final data were presented for the completed open-label extension (OLE) study of ozanimod in adults with RMS (DAYBREAK). Long-term follow-up of participants indicated that the majority remained free of confirmed disability progression (CDP), and a post hoc analysis found no evidence of disease rebound in participants who discontinued ozanimod. Ozanimod was generally well tolerated with sustained efficacy over a treatment period of approximately 6 years, demonstrating a low relapse rate and control of disability progression.
Introduction
Ozanimod is a modulator of spingosine-1-phosphate (S1P) receptor 1 and 5. It is approved in multiple countries for the treatment of adults with RMS or moderately-to-severely active ulcerative colitis.1,2
Four clinical trials of ozanimod in RMS have been completed, including a Phase I pharmacokinetic and pharmacodynamic study,3 a Phase II study with an extension period,4,5 and two Phase III trials.6,7
In the completed Phase III trials (RADIANCE and SUNBEAM), oral ozanimod 0.92 mg/day for up to 24 months was associated with significantly fewer relapses, fewer lesions on brain MRI, and slowed brain volume loss relative to intramuscular IFN β-1a 30 μg/week.6,7 Ozanimod was well tolerated, with fewer treatment-emergent adverse events (TEAE) leading to discontinuation compared with IFN β-1a.
Data from the ongoing Phase III study ENLIGHTEN, and the OLE study DAYBREAK, were presented at ECTRIMS-ACTRIMS 2023 and ACTRIMS 2024.
Interim Analyses from the ENLIGHTEN Study
The ongoing ENLIGHTEN study (NCT04140305)8 is a prospective, multicenter, longitudinal, open-label study in the USA and Canada. It aims to describe changes in cognitive processing speed, disease biomarkers, and patient-reported outcomes, over 3 years, in adults with early RMS treated with ozanimod 0.92 mg.
ENLIGHTEN includes adults aged 18–65 years at screening, with a diagnosis of MS within the last 5 years; ≤1 MS DMT (none within 1 month of enrolment); an Expanded Disability Status Scale (EDSS) score ≤3.5 at screening; no relapses within 30 days before screening; ≤10 gadolinium-enhancing lesions on baseline brain MRI scan; and no history of developmental disorders, or motor or sensory defects, that could interfere with cognitive test performance.8,9
ENLIGHTEN assesses cognitive functioning, in a very early RMS patient population, through the Brief International Cognitive Assessment for Multiple Sclerosis (BICAMS). The BICAMS is a cognitive battery that assesses cognitive processing speed, auditory/verbal learning, and visuospatial memory via the Symbol Digit Modalities Test (SDMT), California Verbal Learning Test Second Edition (CVLT-II), and Brief Visuospatial Memory Test, Revised (BVMT-R), respectively.10,11 Higher test scores indicate greater cognitive functioning.
The primary outcome measure of the ENLIGHTEN study is the proportion of subjects with improvement in SDMT score, defined as an increase in raw score of ≥4 points or 10% from baseline.8 Changes in CVLT-II and BVMT-R scores were exploratory endpoints.12
An ad hoc interim analysis was conducted after 1 year, with a data cut-off of February 14, 2023. Baseline characteristics of participants with data at Year 1 included an average age of approximately 40 years, approximately 79% were female, and the majority (approximately 88%) were White. Roughly 65% of patients were DMT-naïve. Characteristics varied slightly between analyses, depending on the number of participants with available measurements.9,13
Changes in Cognitive Functioning over 1 Year
Impairments in cognitive functioning can begin in the early stages of MS14,15 and are associated with subsequent disease progression and decreased quality of life.16-18
At data cut-off for the ad hoc interim analysis, SDMT data were available at both baseline and Year 1 for 116 participants, CVLT-II data for 102 participants, and BVMT-R data for 93 participants.12
During the first year of ozanimod use, cognitive processing speed remained stable or improved in the majority (73.3%) of participants (Figure 1).13 Almost half of the participants (47.4%) experienced an improvement in cognitive processing speed. While mean SDMT and CVLT-II scores increased from baseline after 1 year of ozanimod treatment, BVMT-R scores remained relatively stable: least squares mean changes in scores were 3.3 for SDMT, 3.2 for CVLT-II, and 0.8 for BVMT-R, generated using a mixed-effects model with repeated measures.12
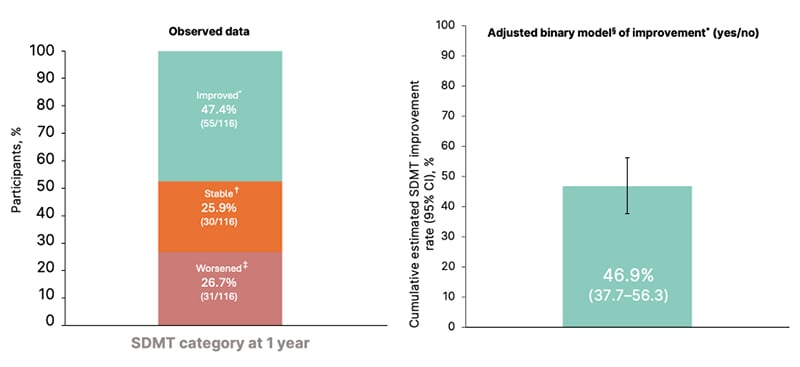
Figure 1: Change in Symbol Digit Modalities Test over 1 year of treatment.
Reproduced with permission from DeLuca et al.12
*Improvement was defined as a ≥4-point or ≥10% increase in SDMT raw score relative to baseline.
†Stable cognitive processing speed was defined as <4 point and <10% change in SDMT.
‡Worsening was defined as a ≥4-point or ≥10% decrease in SDMT.
§Generalized estimating equation model.
CI: confidence interval; RMS: relapsing multiple sclerosis; SDMT: Symbol Digit Modalities Test.
In conclusion, among participants with early RMS, many of whom were DMT-naïve, interim data suggest that ozanimod treatment may prevent cognitive decline during the first year of use.13
In conclusion, among participants with early RMS, many of whom were DMT-naïve, interim data suggest that ozanimod treatment may prevent cognitive decline during the first year of use.13
Brain Volume Changes over 1 Year
While WBV declines with normal ageing at a rate of around 0.27% per year,19 this decline is often accelerated in patients with MS, and may correlate with cognitive impairment.19,20 Alongside these changes, lateral ventricle volume (LVV) increases with atrophy of brain parenchyma in patients with MS.21
The assessment of brain volume using MRI is a secondary outcome of the ENLIGHTEN study.8 At the interim analysis, MRI data were available at baseline and Year 1 for 101 participants.12 The percentage change in WBV and brain region volumes over 1 year were analyzed using the following methods:9
- WBV: Structural Image Evaluation using Normalization of Atrophy (SIENA).
- Cortical grey matter volume (CGMV): A modified hybrid of SIENA and SIENA cross-sectional (SIENAX) methods (mSIENAX multi-timepoint).
- Thalamic volume (TV) and medulla oblongata volume (MOV): FreeSurfer open-source brain imaging software.
- LVV: SIENAX at baseline, and the SIENA method to measure ventricular enlargement (VIENA) on 3D T1-weighted MRI at 1 year.
Over the first year of ENLIGHTEN, observed mean changes in brain volume were minimal: WBV decreased at a rate similar to that seen in healthy controls;19 CGMV, TV, and MOV did not significantly decrease from baseline; and LVV did not significantly increase from baseline (Figure 2).9 It was noted that the observed increase in CGMV may suggest a central effect related to ozanimod S1P receptor selectivity.12
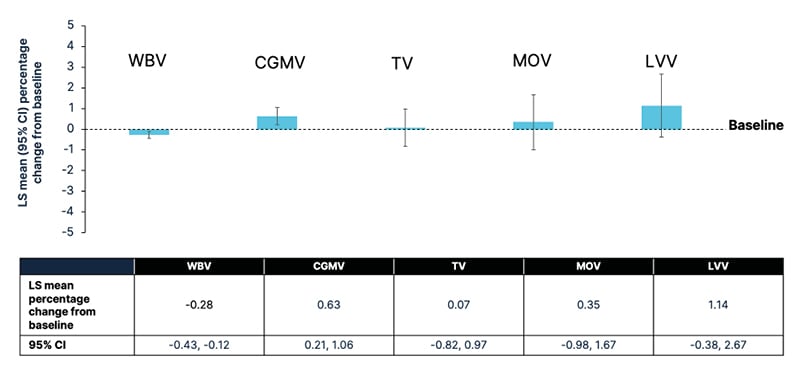
Figure 2: Change in whole brain volume and brain region volumes over 1 year in patients with early relapsing multiple sclerosis treated with ozanimod 0.92 mg.
Reproduced with permission from Reproduced with permission from Zivadinov et al.22
For least squares mean change, a mixed-effect model with repeated measures was used with the baseline volume, baseline age, and time point (treated as a categorical variable) as fixed effects; and individual patients as a random effect.
Kenward and Rogers’ adjustment for the degrees of freedom was applied.
CI: confidence interval; CGMV: cortical grey matter volume; LS: least squares; LVV: lateral ventricular volume; MOV: medulla oblongata volume; RMS: relapsing multiple sclerosis; TV: thalamic volume; WBV: whole brain volume.
In conclusion, these findings indicate that brain volume was preserved during the first year of ozanimod treatment in patients with early RMS.9
New Data from the DAYBREAK Open-Label Extension Study
Participants who completed any of the four RMS trials of ozanimod3-7 were eligible to enroll in DAYBREAK (NCT02576717), a Phase III, single-arm, OLE study of ozanimod 0.92 mg/day.23,24 An interim analysis of the DAYBREAK OLE showed that ozanimod treatment provided sustained control of disability progression for up to 5 years in participants with RMS.25
Relapse-Associated Worsening and Progression-Independent Relapse Activity
There are currently considered to be two main mechanisms for the accumulation of disability during the early stages of MS: relapse-associated worsening (RAW) and progression independent of relapse activity (PIRA).26
The Phase III RADIANCE study (NCT02047734)27 was a multicenter, randomized, double-blind, double-dummy trial comparing oral ozanimod 0.46 mg/day and 0.92 mg/day with intramuscular IFN β-1a 30 μg/week in adults with RMS.23 Primary findings from the study indicated that ozanimod treatment was associated with significantly fewer relapses than IFN β-1a.6
In a long-term analysis of participants from the RADIANCE Phase III study who entered DAYBREAK (data cut-off: February 1, 2022), the two treatment groups were those who received continuous ozanimod 0.92 mg/day (n=363) across both studies and those who switched from IFN β-1a to ozanimod 0.92 mg/day (n=346) upon entering the DAYBREAK OLE. Data from this analysis, reported at the 2023 ECTRIMS-ACTRIMS meeting, showed that the two treatment groups had similar demographic and baseline disease characteristics, with a mean age of approximately 36 years. Around 68% of participants were female, and approximately 99% were White.22
Participants were assessed for 6-month CDP, defined as a ≥1-point increase in EDSS score from baseline confirmed at 6 months.23 Participants were considered to have RAW if CDP onset occurred ≤90 days after a relapse, or to have PIRA if CDP onset occurred without relapse or >90 days after a relapse. For any relapse with incomplete recovery, baseline EDSS was reset >90 days after relapse onset, to allow for identification of the next PIRA event.23 In addition, WBV and CGMV were measured using SIENAX, and TV using ThalamicVolume software.22
On long-term follow-up (up to 8 years), approximately 76% of all participants remained free of 6-month CDP (Figure 3).22 Overall, data indicated that RAW and PIRA contributed similarly to disability progression. Of those participants with 6-month CDP who were treated with continuous ozanimod, 44.3% had RAW, 54.5% had PIRA, and 8.0% had both, relative to RADIANCE baseline. 23 Of those participants with 6-month CDP who switched from IFN β-1a to ozanimod, 57.8% had RAW, 43.4% had PIRA, and 4.8% had both, relative to RADIANCE baseline.23
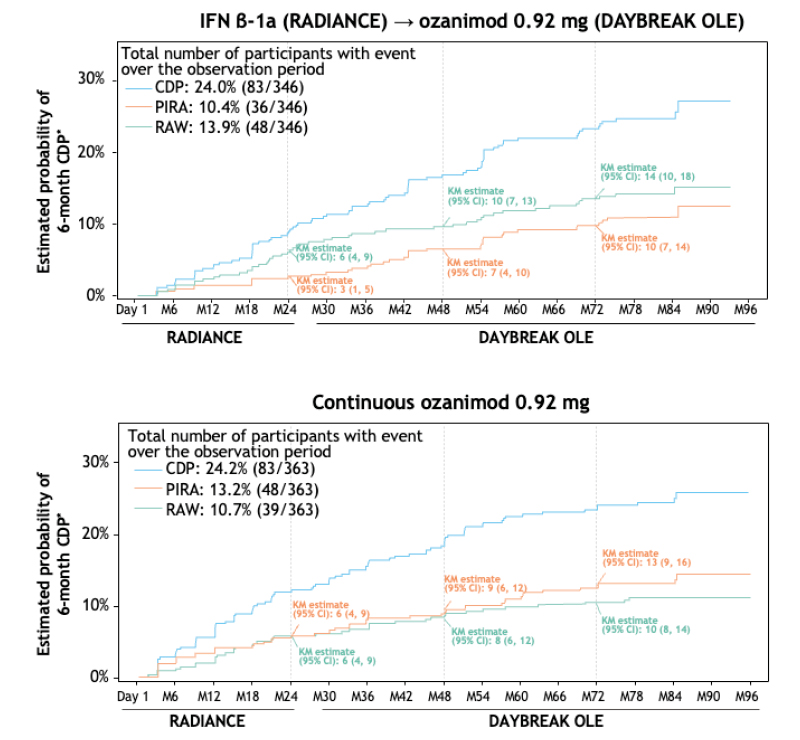
Figure 3: Time to onset of sustained disability progression: 6-month confirmed disability progression during RADIANCE and the DAYBREAK open-label extension.
*Kaplan-Meier analyses of the intention-to-treat population.
CDP: confirmed disability progression; IFN: interferon; KM: Kaplan-Meier; M: month; PIRA: progression independent of relapse activity; OLE: open-label extension; RAW: relapse-associated worsening.
Relative to baseline at enrolment in the DAYBREAK OLE, participants who were treated with continuous ozanimod experienced numerically less 6-month CDP compared with participants who switched from IFN β-1a (14.0% versus 17.9%, respectively). In addition, among patients treated with continuous ozanimod, the proportion of PIRA contributing to 6-month CDP was higher relative to DAYBREAK baseline (70.6%) than in the analysis relative to RADIANCE baseline (54.5%).22
In both treatment groups, EDSS at RADIANCE baseline was positively associated with the incidence of RAW; and WBV, CGMV, and TV thalamic volumes were negatively associated (P<0.05). However, these variables were not found to be predictors of PIRA.23
In conclusion, these data suggest that while controlling relapses is essential early in the MS disease course, other processes such as lesion development and brain volume loss may also be important.22
Absence of Rebound Effect Following Ozanimod Discontinuation
Rebound is described as a severe relapse of disease following discontinuation of therapy. Discontinuation of MS DMT has been associated with a risk of disease reactivation, particularly if treatment with another DMT is not initiated, and some cases have been reported in which increased MRI activity has occurred.28-32 To assess the risk of rebound (characterized by severe disease reactivation and physical disability) after ozanimod discontinuation, an exploratory, post hoc analysis was conducted on data from the DAYBREAK OLE study (database lock April 7, 2023).33
Participants were included in the analysis if they had at least 1 day of post-treatment safety follow-up in DAYBREAK, and they did not transition to commercial ozanimod within the safety follow-up period (initially 28 days but increased to 75 days in 2018 and 90 days in 2019).12 A total of 1,679 participants were included, of whom 55 (3.3%) had a known post-discontinuation relapse. The mean ozanimod exposure during DAYBREAK was 61 months.33
This analysis found no evidence of rebound in participants who permanently discontinued ozanimod: only one patient relapse was reported as severe by the investigator, and most patients who relapsed had an EDSS category change of 0.5 (40.0%) or 1.0 (25.5%). Relapse predominantly occurred during the second and third months after ozanimod discontinuation (median time from last dose to relapse: 61 days), was most common in untreated participants, and was generally mild or moderate.33
In conclusion, most DAYBREAK participants did not have MS disease reactivation characterized by relapses within 90 days of permanent ozanimod discontinuation.
Long-Term Efficacy and Safety of Ozanimod
The DAYBREAK OLE study was completed in January 2023, and final safety and efficacy results of extended exposure to ozanimod were reported at the ACTRIMS Forum (database lock: April 7, 2023).12
Of 2,494 participants who enrolled in DAYBREAK and received at least one dose of ozanimod 0.92 mg, 1,950 (78.2%) completed the study.33,34 Of the 21.8% of participants who discontinued treatment during the study, 14.6% discontinued due to the Russian invasion of Ukraine, and 3.9% discontinued due to TEAEs.12,34 The mean age of participants was 37.7 years, 67% were female, and 99% were White. Baseline demographics and disease characteristics were generally consistent across parent trial treatment groups.12 The mean exposure to ozanimod in DAYBREAK was 61 months;34 while the mean exposure to any dose of ozanimod across the parent trials and DAYBREAK was 75 months, with a maximum of 117 months.12
Ozanimod treatment demonstrated sustained efficacy, with most participants remaining relapse free during DAYBREAK. At Months 60 and 72, 69% and 67% of participants, respectively, were relapse free. Participants who continuously received ozanimod through both a parent trial and DAYBREAK experienced the lowest annualized relapse rate (ARR) compared to other groups. The greatest numerical reduction in ARR from the Phase III parent trial to DAYBREAK occurred among those who switched from IFN β-1a to ozanimod 0.92 mg.12
During DAYBREAK, 17.2% of participants had 3-month CDP and 15.2% had 6-month CDP by the end of the study.34 Across the Phase III parent trials and DAYBREAK together, 22.5% of participants had 3-month CDP and 19.8% had 6-month CDP by the end of DAYBREAK.12
The majority (89.0%) of participants experienced a TEAE during DAYBREAK; 15.3% had a serious TEAE (SAE), and 3.9% discontinued due to a TEAE. Similar rates of TEAEs and serious TEAEs occurred when assessed by the parent trial treatment group.34
Two participants died due to malignancies during the study; two due to accidents; two due to pulmonary embolism; and one each due to abscess of the right lung, COVID-19, COVID-19 bilateral pneumonia, COVID-19 infection, COVID-19 pneumonia, heart failure, intracerebral hemorrhage, pneumonia, and sudden death.12
Infections were reported in 64% of participants, most commonly nasopharyngitis (21.3%), COVID-19 (16.5%), and upper respiratory tract infection (12.4%); 4.3% of participants experienced a serious infection. Opportunistic infections were reported in 6.4% of participants, most commonly oral herpes (2.3%) and herpes zoster infections (2.0%). Only one serious opportunistic infection occurred during DAYBREAK (progressive multifocal leukoencephalopathy)12,25
Macular edema was reported in 10 (0.4%) participants, of which five cases were confirmed by the Macular Edema Review Panel. Cardiac TEAEs occurred in 103 (4.1%) DAYBREAK participants, 11 of whom had serious events. Absolute lymphocyte count (ALC) <0.2×109 /L was reported in 369 of 2,488 participants with available ALC data, of whom 44 had a consecutive ALC <0.2×109 /L on retest within 30 days.12
Across the parent trials and DAYBREAK together, 50 (1.8%) participants exposed to either dose of ozanimod developed treatment-emergent malignancies, including one with malignant melanoma (0.04%), 17 non-melanoma skin cancers (0.6%), and 31 non-cutaneous malignancies (1.1%).12
In conclusion, ozanimod demonstrated sustained efficacy over a treatment period of approximately 5 years, with a low relapse rate, low numbers of new/enlarging lesions on brain MRI, and control of disability progression. Ozanimod is generally well tolerated, with a small percentage of patients discontinuing treatment due to TEAEs, and it continues to demonstrate a strong safety profile over 5 years1,2,6,7,12