Meeting Summary
This symposium took place during the 2023 Congress of the European Academy of Neurology (EAN). Mar Carreño, Director, Epilepsy Unit, Hospital Clínic and Instituto Clavel, Barcelona, Spain, presented the definition of drug-resistant epilepsy (DRE), and stressed that uncontrolled epilepsy does not necessarily indicate DRE. Before a diagnosis of DRE is made in a patient not responding to medication, questions should be asked regarding the initial epilepsy diagnosis. Carreño discussed paroxysmal events that may mimic epilepsy, and presented three cases of misdiagnosed DRE that were subsequently correctly identified as cardiac syncope, a psychogenic event, and use of inappropriate medication in a patient with generalised epilepsy. The second part of Carreño’s presentation focused on patients with confirmed DRE. They outlined the complications of DRE, including sudden unexpected death in epilepsy (SUDEP), which should be discussed with the patient. Carreño finished their lecture with a discussion of comorbid conditions, including neuropsychiatric comorbidities, which affect one in three patients with epilepsy. Bernhard J. Steinhoff, Medical Director, Kork Epilepsy Center, Kehl, Germany, then discussed the clinical approach to patients with DRE, including treatment options, the range of anti-seizure medications (ASM), and the reasons for failure of first-line treatment, noting that the probability of achieving seizure freedom decreases with each failed ASM. Steinhoff explored the options of substitution monotherapy or combination therapy after failure of the first ASM, before describing cenobamate (CNB) add-on therapy. A randomised, placebo-controlled, dose-response trial showed that adjunctive CNB reduced focal (partial)-onset seizure frequency in a dose-related fashion. Several papers have been published providing real-world evidence to show that adjunctive CNB therapy is associated with improved seizure outcomes, and that the number of concomitant ASMs could be reduced. The symposium concluded with a question and answer session.
A Spectrum of Uncontrolled Epilepsies
Mar Carreño
Definition of Drug-Resistant Epilepsy
Carreño began by discussing the spectrum of uncontrolled epilepsies. They stressed that uncontrolled epilepsy does not necessarily indicate DRE, and noted that this is important in ensuring that the correct treatment is offered. According to the International League Against Epilepsy (ILAE), patients are considered to have DRE if they are not seizure free after administration of two appropriate ASMs as sequential monotherapies or as combination therapy.1
Sustained seizure freedom is defined as freedom from seizures for at least three-times the longest inter-seizure interval before medical intervention (determined for seizures occurring in the previous year), or 12 months, whichever is longer.1 If duration of seizure freedom is <12 months, treatment outcomes should be classified as undetermined. If the patient has another seizure before the end of the 12-month period, the treatment is considered failed, even though the seizure frequency has reduced compared with baseline.1
Checking the Initial Diagnosis of Epilepsy
Before a diagnosis of DRE is made in a patient not responding to medication, Carreño suggested, based on their own experience, that the following questions should be asked regarding the initial diagnosis of epilepsy: is it a seizure or another type of paroxysmal event?; is it really the first seizure, or have other seizures occurred previously that went unnoticed? (this situation is very common with absence seizures and myoclonic seizures); is it an acute symptomatic seizure or an unprovoked seizure?; what is the risk of recurrence?; and, in cases where a diagnosis of epilepsy is highly likely, what type of epilepsy is it? This will determine the type of treatment required.
Carreño has observed in their own clinical practice that a number of paroxysmal events may mimic epilepsy. These include neurological conditions such as migraine (particularly migraine with aura), transient global amnesia, movement disorders (paroxysmal dystonia), parasomnias, and metabolic disturbances. In clinical practice, Carreño has found that the conditions that most frequently mimic epilepsy are psychogenic seizures and syncope.
As physicians rarely observe first seizures, the diagnostic process should include careful history-taking2 with a reliable witness, especially if the patient reports loss of consciousness. If the patient is experiencing repeated episodes, it is important to establish if there are stereotypical features of epilepsy. Potential triggers should be identified, as these may be different in cases of syncope, for example. Physicians should check for signs and symptoms suggestive of epilepsy, including premonitory symptoms, aura, postictal confusion, drowsiness, headache, or myalgia. Home video recordings should be obtained when possible and reviewed. Postictal and interictal investigations, such as electroencephalogram (EEG) and neuroimaging tests, should also be performed. Video EEG monitoring may be required.
Case of Cardiac Syncope
Carreño discussed the case of a 72-year-old female referred to Carreño’s centre with DRE. The patient’s seizures were classified as complex partial seizures, with oral automatisms and secondary generalisation causing repeated falls and several fractures. They underwent video monitoring, which revealed shallow breathing, loss of awareness, neck extension with open eyes, stiffness of the right arm, and movements of the mouth. The patient did not respond to treatment with carbamazepine (CBZ) and levetiracetam (LEV).
An EEG/ECG recorded during the episode demonstrated bradycardia followed by asystole. During asystole, the EEG showed a generalised flattening relating to hypoperfusion with muscle artefacts due to tonic extension of the body. The patient was discharged with a pacemaker, and antiepileptic drugs were discontinued. The patient did not have DRE or epilepsy.
Case of a Psychogenic Event
Carreño presented a second case of a male undergoing invasive monitoring with subdural electrodes. They had a partial seizure with head and eye movements and sustained deviation to the left, followed by deviation of the trunk, stiffening, and jerking of the left arm and face. The aetiology of the seizure was right frontal cortical dysplasia. The patient underwent surgery and was subsequently seizure free for 6 months.
After 6 months, the patient presented to the emergency department with recurrent seizures, despite compliance with medication. Monitoring demonstrated irregular jerking of the head, and head and trunk deviation to the left with preservation of consciousness. An EEG showed no change, suggesting a psychogenic event in a patient with prior epilepsy.
Case of Inappropriate Medication
Another potential reason for the mistaken diagnosis of DRE is inappropriate use of medication. A third case was presented, describing a 23-year-old female with seizure onset at 10 years of age. The patient reported head jerks, head deviation to the right, and generalised jerking. In addition, the patient had seizures with brief loss of awareness several times a week. An EEG had been performed in another centre, which showed left frontocentral focal epileptiform activity. Left frontocentral epilepsy was suspected, and the patient was referred to Carreño for presurgical evaluation. The patient was resistant to CBZ, oxcarbazepine, gabapentin, and lacosamide (LCM). The patient was admitted for video EEG, which showed interictal generalised interictal discharges.
The recorded seizure consisted of jerks and eye deviation to the left (rather than the right, as the patient had claimed), left face and arm jerks, and a generalised tonic phase followed by generalised clonic jerking. The corresponding EEG was generalised from the onset, and the patient was diagnosed with juvenile myoclonic epilepsy (JME). The patient had primarily been receiving sodium-channel blockers (SCB), which may worsen seizures in patients with JME. It was noted that focal features may be seen in seizures and EEG of patients with generalised epilepsies.
Seizure Aggravation by Use of Incorrect Anti-Seizure Medications
Certain drugs, especially SCBs such as CBZ, can both aggravate and induce new seizure types in absence epilepsy, JME, and other genetic generalised epilepsies.3 In other epilepsies, CBZ may aggravate myoclonic, atonic, or atypical absence seizures. CBZ often causes new or more severe generalised paroxysmal abnormalities on an EEG, and this may correlate with seizure aggravation.3
Vigabatrin may aggravate absence seizures in childhood absence epilepsy, and may exacerbate or induce myoclonic seizures in myoclonic epilepsies.3 Lamotrigine (LTG) may aggravate myoclonic seizures, while gabapentin may exacerbate absence epilepsy and myoclonic seizures.3
Importance of a Correct Diagnosis
A false positive diagnosis of DRE can have severe psychological and socioeconomic consequences for the patient, including unnecessary driving restrictions and employment difficulties.4 Patients may experience iatrogenic harm due to inadequate intake of ASMs. In addition, some life threatening conditions may be missed, including cardiac syncope for example.
Complications of Drug-Resistant Epilepsy
The second part of Carreño’s presentation focused on patients with confirmed DRE. They pointed out that patients with DRE have seizures and numerous complications, which pose a significant healthcare challenge. Patients are at risk of injury and premature mortality.5 The majority of deaths are epilepsy-related, and 40% are due to SUDEP.6
Sudden Unexpected Death in Epilepsy
SUDEP is 40-times more likely in patients with ongoing seizures than in those who are seizure free,7 and is the most common cause of premature death among individuals with epilepsy.8,9 Although the precise cause of SUDEP remains unknown, it is thought to include a central mechanism involving cardiac and respiratory dysfunction after a generalised tonic-clonic seizure (GTCS), and a brainstem mechanism involving adenosine and serotonin.8 Consistent risk factors include poor seizure control; frequent GTCS, especially at night; and long-standing epilepsy.9 Deaths are usually nocturnal and unwitnessed.10
In Carreño’s experience, the most effective strategy for prevention of SUDEP is complete seizure control, but if the patient’s epilepsy is uncontrolled, treatment with new ASMs should be attempted. They added that seizure precipitants should be avoided, and adherence to chronic treatment should be encouraged. Devices are recommended to detect and alert caregivers about the occurrence of nocturnal GTCS, as adequate supervision and stimulation may decrease the incidence of SUDEP.11
Comorbid Conditions
Comorbid conditions are more common in patients with epilepsy than in the general population.12 Common comorbidities include cerebrovascular accidents, dementia, gastrointestinal and digestive disorders, migraine, musculoskeletal system disorders, depression, and anxiety.12
Neuropsychiatric Comorbidities
Neuropsychiatric comorbidities affect one in three patients with epilepsy.13 Simple and standardised screening tools are available to diagnose neuropsychiatric comorbidities in patients with epilepsy, for example, the Neurological Disorders Depression Inventory for Epilepsy (NDDI-E).14 Neuropsychiatric comorbidities often complicate the diagnostic process, as depression, for example, may mimic seizures or adverse events caused by ASMs.15 Neuropsychiatric comorbidities also influence the response to treatment.16 The severity of depression correlates with a lower odds of achieving seizure remission.12 Psychiatric disease is associated with premature mortality, increased risks of substance or alcohol abuse, increased risks of injury, and increased rates of suicide and SUDEP.17
Carreño recommended that in patients with psychiatric conditions, ASMs with potential psychiatric side effects should be avoided. They advised that when discontinuing mood stabilising agents, patients should be warned about psychiatric symptoms. In their experience, antidepressant and antianxiety drugs should be prescribed, such as selective serotonin reuptake inhibitors, taking into account possible interactions with ASMs.
Conclusions
Uncontrolled epilepsy cannot always be classified as DRE. In difficult cases, physicians should consider whether the diagnosis may be incorrect, or whether the patient may be receiving an inappropriate treatment. In patients with confirmed DRE, the impact of comorbid conditions should be considered. In particular, psychiatric conditions may lead to premature mortality and a reduced quality of life, and patients should be screened, treated, and referred for psychiatry assessment if current treatment is unsuccessful. At-risk patients should be made aware of the risk of SUDEP, and the importance of adhering to treatment.
The Clinical Approach
Bernhard J. Steinhoff
Treatment Options for Epilepsy
Steinhoff opened their presentation by stating that the ultimate goal of epilepsy treatment is apparent seizure freedom without any treatment-emergent adverse events. Steinhoff commented that current treatment options include lifestyle adaptation, which may be particularly difficult in adolescence; chronic ASM; epilepsy surgery; neurostimulation; and dietary therapies. Chronic ASM is the gold standard for almost all patients.
Anti-seizure Medications
The development of ASMs began in the 19th century with the first use of bromide to manage seizures.18 Today, fourth-generation ASMs provide more options for patients, although questions remain over how these medications should be used to increase the proportion of patients achieving sustained freedom from seizures. In Steinhoff’s opinion, the criteria for selecting first-line medication include the pharmacokinetics of the drug, its spectrum of efficacy, interactions with other medications, acute and long-term tolerability, and potential teratogenicity.
The SANAD II trial was designed to assess non-inferiority of LEV and zonisamide (ZNS) to LTG for the primary outcome of time to 12-month remission.19 In the intention-to-treat analysis of time to 12-month remission versus LGT, LEV did not meet the criteria for non-inferiority but ZNS did.19 In the per-protocol analysis, 12-month remission was superior with LTG compared with both LEV and ZNS.19 The trial demonstrated that 12-month remission was superior with LTG compared with both LEV and ZNS in patients with focal epilepsy, indicating that LTG should remain a first-line treatment for this patient group.19 The risk of taking ASM during pregnancy also needs to be considered, with the potential for congenital malformation following prenatal exposure.20 The International Registry of Antiepileptic Drugs and Pregnancy (EURAP) registry showed a 10.3% rate of congenital malformations with valproic acid (VPA), and a dose-dependent risk increase among the children of females who had been exposed to ASM during pregnancy.20 In contrast, the rate of malformations was 2.8% with LEV, with no dose-dependent risk increase.
Failure of First-Line Treatment
In Steinhoff’s experience, the reasons for failure of first-line treatment include tolerability, for example, rash with LTG; irritability and sedation with LEV; and obesity, hair loss, and tremor with VPA. They said that after failure of first-line treatment, the physician should examine whether the initial diagnosis was incorrect, and the patient had instead experienced psychogenic seizures, syncope, or other paroxysmal events. In addition, Steinhoff stated that the physician should also check whether the classification of epilepsy was correct, as some ASMs may trigger, rather than prevent, seizures. For example, LTG may trigger myoclonic seizures, while CBZ, oxcarbazepine, and eslicarbazepine acetate (ESL) may trigger absence or myoclonic seizures. Steinhoff noted that adherence to medication should be checked and, in some cases, a drug with another mode of action could be tried. Another question, they added, is whether to proceed with alternative monotherapy, or with combinations of treatments.
The underlying cause of epilepsy is a major prognostic factor for recurrence.21 An observational survey of 2,200 adult outpatients with partial epilepsy showed that seizure control (>1 year without seizure) was achieved in 82% of patients who had idiopathic generalised epilepsy, 35% of patients with symptomatic partial epilepsy, 45% with cryptogenic partial epilepsy, and 11% with partial epilepsy associated with hippocampal sclerosis.21 In partial epilepsy, dual pathology (hippocampal sclerosis and another lesion) was associated with a low rate of freedom from seizures (3%).
Substitution Monotherapy or Combination Therapy After Failure of the First Anti-seizure Medication
Currently there are a number of ASMs that might be combined without drug interactions. According to the longitudinal observational study in people with epilepsy after failure of initial monotherapy reported by Hakeem et al.,22 seizure outcomes were similar on substitution or combination therapy, and the type of ASM used, either alone or in combination, did not affect seizure outcome. Similarly, a prospective observational study among children and adults with epilepsy in whom first monotherapy failed, showed that retention time, hospital admissions, days off work and off school, and quality of life did not differ between patients on monotherapy or combination therapy.23 A further observational study also showed that the seizure remission rate and retention rate of substitution therapy were better than those of add-on therapy for patients with focal epilepsy whose first monotherapy failed.24 The choice of substitution monotherapy or combination therapy therefore requires an individualised approach.
Achieving Seizure Freedom
Steinhoff next presented data to show that although around two-thirds of patients achieve seizure freedom with ASMs, the probability of success decreases with each failed treatment. For example, in 2000, Kwan and Brodie25 demonstrated that among 470 previously untreated patients with newly diagnosed epilepsy, 222 (47%) became seizure free during treatment with their first ASM, and 14% became seizure free during treatment with a second or third drug. For 12 patients (3%), epilepsy was controlled with two drugs. Chen et al.26 showed in 2018 that despite the availability of new ASMs with different modes of action, the probability of seizure freedom decreased with each subsequent ASM tried, and that more than one-third of patients have uncontrolled epilepsy.
The evidence concerning seizure freedom with add-on treatment is restricted to meta-analyses, with no direct comparative trials between the newer ASMs. A network meta-analysis of brivaracetam (BRV), CNB, ESL, LCM, and perampanel (PER) showed that all ASMs were associated with a higher responder rate than placebo, while BRV, CNB, ESL, and PER were associated with a higher rate of seizure freedom than placebo.27 CNB ranked highest for efficacy, with a greater rate of ≥50% seizure frequency reduction than BRV, ESL, LCM, or PER.27
Villanueva et al.28 investigated the number needed to treat to achieve a response or freedom from seizures among patients with DRE. The authors concluded that CNB may be the most effective ASM in all doses studied compared with third-generation ASMs, as well as the most efficient option at the daily defined dose for both 50% responder rate and seizure freedom.28 Steinhoff noted that the study could contribute to informed decision-making regarding the selection of the most appropriate therapy for focal onset seizures (FOS) in adult patients with DRE.
Cenobamate Add-on Therapy
CNB has a dual mode of action, exerting its effects by reducing excitatory currents (mainly by inhibition of persistent sodium channels) and augmenting inactivated sodium channels.29 It is also a positive allosteric modulator of the γ-aminobutyric acid type A-receptor at the nonbenzodiazepine binding site.30 The long half-life of CNB permits once-daily dosing, which is beneficial for adherence.31,32 On the other hand, the drug has a complex metabolism, and interactions with other drugs, including other ASMs, need to be considered.32
A randomised, placebo-controlled, dose-response trial in patients with uncontrolled focal seizures showed that adjunctive CNB reduced focal (partial)-onset seizure frequency in a dose-related fashion.33 Freedom from seizures was achieved by 21% of patients in the CNB 400 mg group, 11% in the CNB 200 mg group, 4% in the CNB 100 mg group, and 1% of those on placebo.33 Several papers have also been published presenting real-world evidence to show that adjunctive CNB is associated with improved seizure outcomes.34-36
Real-World Evidence with Cenobamate
A multicentre, retrospective, observational study was conducted to assess the safety and effectiveness of CNB in patients with refractory epilepsy in a real-world setting.36 The study included 170 patients from the Early Access Programme in Spain, which enrolled patients with no other therapeutic alternative. Participants had taken an average of 12 previous ASMs, 36 patients had undergone vagus nerve stimulation therapy, and 34 had received resective surgery. Patients were taking an average of three concomitant ASMs. Mean CNB dosages/day were 176 mg, 200 mg, and 250 mg at 3, 6, and 12 months, respectively.
Results of the study confirmed the efficacy data from pivotal clinical trials (Figure 1).36 In a post hoc analysis, 18.1% of patients were continuously seizure free during follow-up for at least 3 months, and 19.5% of patients were continuously seizure free during follow-up for at least 6 months.
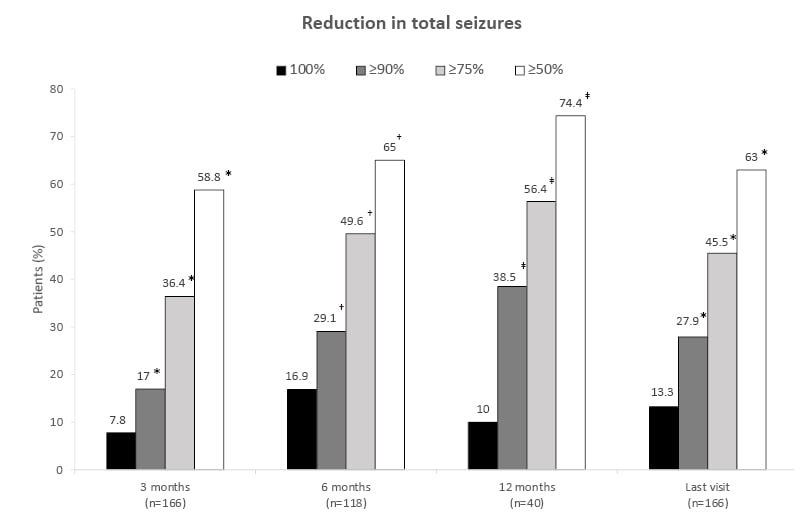
Figure 1: Real-world data demonstrates the effectiveness of cenobamate.36
*n=165
†n=117
‡n=39
Proportion of patients with a reduction in the frequency of total seizures following cenobamate treatment. Reductions in seizure frequency are displayed as a 100% reduction from baseline or a ≥90%, ≥75%, or ≥50% reduction since the previous patient visit. Patients with a ≥50% reduction from the previous visit are considered responders at that timepoint.
Adapted from Villanueva et al.36
When the effectiveness of CNB was examined according to the number of previous ASMs, the high response rate in patients who had already taken at least six ASMs, which is considered absolute drug resistance, could suggest a breakthrough response in this patient profile (Figure 2). Although only a small number of patients received early treatment, the data suggest improved efficacy when CNB is initiated earlier in the course of the disease.
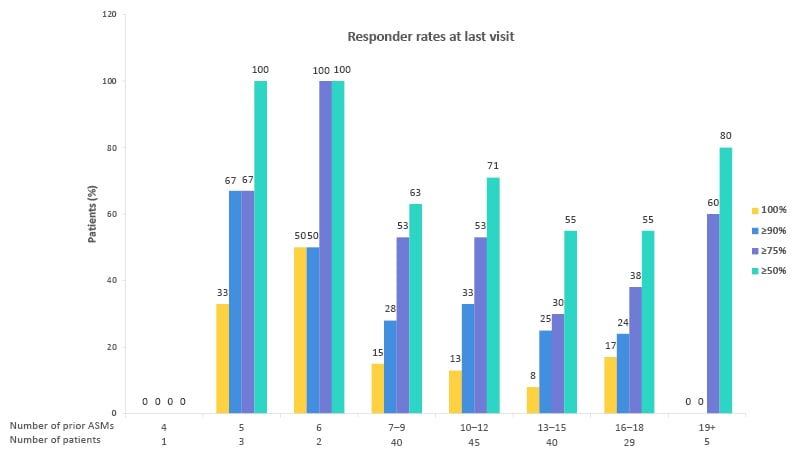
Figure 2: Effectiveness of cenobamate by number of previous anti-seizure medications.36
Responder rates at last visit.
Proportion of patients with an improvement in seizure frequency at the last visit by the number of prior ASMs. Reductions in seizure frequency are displayed as a 100% reduction from baseline, or a ≥90%, ≥75%, or ≥50% reduction since the previous patient visit. Patients with a ≥50% reduction from the previous visit are considered responders at that timepoint.
Adapted from Villanueva et al.36
ASM: anti-seizure medication.
This real-world study also demonstrated that in patients who benefitted from CNB, there was a trend towards reduction in baseline medication (Figure 3).36 Some 44.7% of patients reduced the number of concomitant ASMs required, with primary reasons including the efficacy of CNB, and the avoidance of pharmacodynamic side effects due to drug interactions. SCBs and clobazam were the most commonly reduced ASMs. CNB increases the serum concentration of the main metabolite of clobazam, which may cause side effects.
The percentage, type, and severity of adverse events in this real-world study were similar to those reported in clinical trials.36 The main adverse events with add-on CNB were central nervous system-related, with somnolence occurring in 35.9% of patients and dizziness in 26.5%. Retention rates also reconfirmed the data from clinical trials.36 At 12 months, the retention rate was 87%, supporting the clinical benefit of CNB in adults with uncontrolled FOS.
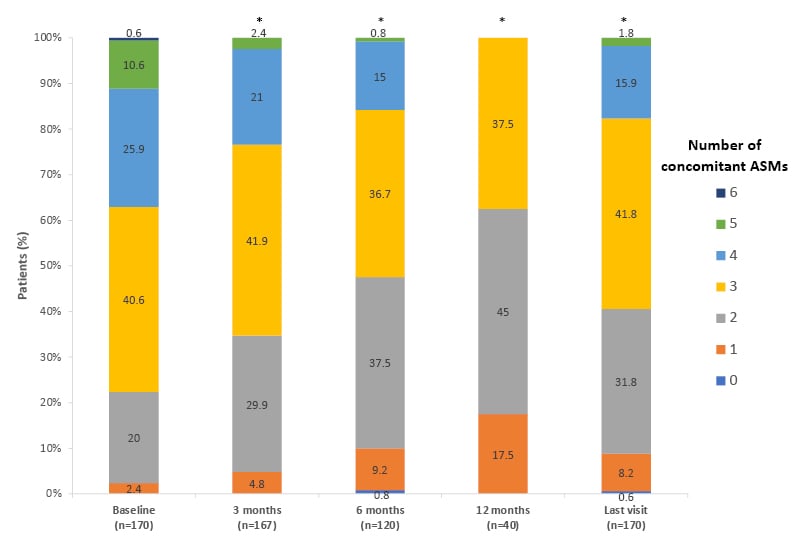
Figure 3: Reduction of concomitant anti-seizure medications with cenobamate.36
Proportion of patients receiving concomitant ASMs following cenobamate treatment.
Bars show the proportion of patients receiving 0–6 concomitant ASMs at each timepoint.
*p<0.001 versus baseline.
Adapted from Villanueva et al.36
ASM: anti-seizure medication.
Case of Cenobamate Therapy
Steinhoff presented the case of a board-certified neurologist who was born in 1969, and had been involved in a car accident at 13 years of age. They had a right temporal epidural haematoma followed by a left temporal abscess, hemiparesis, aphasia, and hemianopia. The patient had impaired attention and memory, but was still able to obtain a high school diploma and graduate from university. Following the onset of epilepsy in 1994, the patient experienced drug-resistant structural FOS with focal aware acoustic and olfactory seizures, focal unaware seizures, focal to bilateral tonic-clonic seizures, and intermittent depressive episodes with suicidal ideations.
The patient demonstrated ideal adherence to medication, with a history of 11 ASM drugs: CBZ, VPA, LTG, topiramate, LEV, LCM, ZNS, phenytoin, ESL, PER, and BRV. An MRI demonstrated that the risk of resective epilepsy surgery would have been extremely high; therefore, surgery was not pursued. An interictal EEG showed intermingled spikes over the left temporal region. The patient is now receiving CNB 350 mg per day, and both citalopram and LEV have been discontinued. During an observation period of >2 years, they have had less than one focal aware acoustic seizure per month. The patient has no abnormal ECG or laboratory findings, and their quality of life has improved.
Conclusions
Steinhoff concluded by remarking that there are a number of options available today to overcome the burden of DRE. Clinical trial evidence has demonstrated that adjunctive CNB reduces seizure frequency in a dose-related fashion. In addition, real-world evidence has demonstrated that adjunctive CNB is associated with improved seizure outcomes, and a reduction in the number of concomitant ASMs.
Question and Answer Session
Bernhard Steinhoff and Mar Carreño
The symposium concluded with a question and answer session. Carreño was asked why they would prescribe citalopram or sertraline as opposed to the other selective serotonin reuptake inhibitors for the treatment of depression in patients with epilepsy. Carreño explained that these medications are well-tolerated, widely available, and effective in patients with a combined profile of anxiety and depression.
Steinhoff was asked about surveillance of patients taking CNB. They said that their laboratory measures levels of CNB and other ASMs in order to understand potential drug interactions in individual cases. Steinhoff’s hospital also measures liver enzyme concentrations, although elevations are infrequent. They also perform ECGs, but noted that no cardiological problems have been found in more than 500 patients with add-on CNB.







