Meeting Summary
The two most common forms of non-dystrophic myotonia (NDM) are myotonia congenita (Thomsen disease or Becker-type) and paramyotonia congenita. Symptoms, including muscle stiffness, cramps, and transient weakness, can affect a person’s quality of life. An unmet need for a validated tool to assess myotonia symptom severity and frequency, as well as disability caused by myotonia, led to the development of the Clinical Myotonia Rating Scale (CMRS). At the 9th Congress of the European Academy of Neurology (EAN), Budapest, Hungary, 1st–4th July 2023, a poster was presented regarding validation and reliability testing of the CMRS, the results of which are discussed here. Such a tool is needed when first assessing myotonia symptoms in a patient with NDM, as well as when assessing their response to myotonia-targeting medication. One such drug is mexiletine, a Class 1B antiarrhythmic agent that is approved for the treatment of myotonia symptoms in adults with NDM. Although a number of studies, including clinical and real-world trials in people with NDM, have not found mexiletine to be associated with impaired cardiac function, but as an antiarrhythmic drug, cardiac assessment is required with mexiletine prescription. Also presented at the 2023 EAN meeting is an algorithm to aid prescribers in understanding patients in whom mexiletine may be contraindicated, tests needed prior to mexiletine prescribing, and cardiac monitoring under treatment in patients with NDM. This algorithm was developed utilising expert opinion, the mexiletine summary of product characteristics, and a literature review of mexiletine safety data in NDM.
Introduction
In the rare neuromuscular disorders classed as NDM, muscle stiffness (myotonia) is the major symptom and is defined by a delayed muscle relaxation after voluntary contraction.1 In myotonia congenita (Thomsen disease or Becker-type), myotonia occurs due to loss-of-function mutations in the CLCN1 gene that codes for the chloride channel CLC-1.1-3 In paramyotonia congenita, myotonia occurs due to gain-of-function mutations in the SCN4A gene that codes for the Nav1.4 sodium channel.1,4 Depending on subtype, individual symptom severity, and exacerbating factors, a patient with NDM may also experience cramps, transient weakness, myalgia, fatigue, muscle hypertrophy, speech, chewing and swallowing difficulties5,6 In the IMPACT survey, including 181 adults with NDM and 59 carers, respondents reported how NDM symptoms could physically, socially, and psychologically impact their quality of life.6
Development of the Clinical Myotonia Rating Scale
Measures that can help assess myotonia include the modified Myotonia Behaviour Scale (MBS),7 the Timed Up and Go (TUG) test,8 and Borg Category-Ratio scale.9 However, as there are no standardised and validated tools specific to NDM, the CMRS was developed on the model of the Dystonia Movement Scale and Disability Scale10 to help assess a range of NDM-associated symptoms with regard to severity and disability (Figure 1).11
In a poster by Savine Vicart, Muscle Channelopathies Reference Center, Assistance Publique-Hopitaux de Paris (APHP), University Hospital Pitié-Salpêtrière, Paris, France, and colleagues, a tool was discussed that can help objectively in assessing myotonia in routine clinical practice.12 The CMRS comprises a Myotonia Severity Scale (MSS), which assesses and scores myotonia severity and frequency in six body areas, and a Disability Scale (DS), which scores myotonia severity in seven daily activities (Figure 1). For the MSS, frequency is scored from 0 (none) to 4 (every day). Severity is scored from 0 (none) to 4, according to each area, for example, for eyelids and limbs, a 4 rating is up to ‘severe, permanent’; for ocular muscles, it is ‘severe, diplopia’; for chewing and swallowing, it is ‘unable to chew, choking’; and for respiratory muscles, it is ‘permanent dyspnoea.’ Total MSS score is calculated based on severity and frequency with a weighting factor for each component, and a total range of 0−104.11
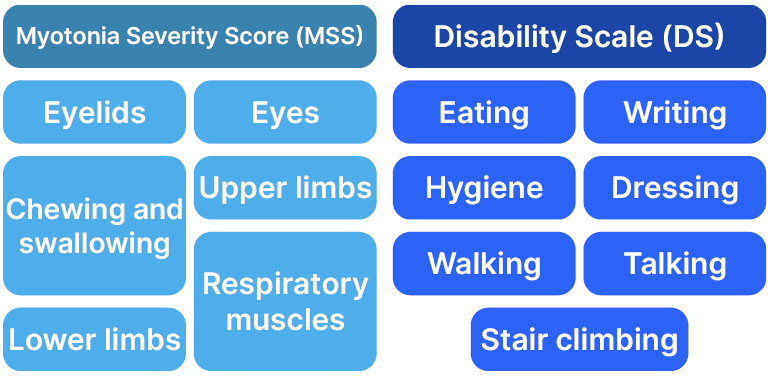
Figure 1: Components of the Clinical Myotonia Rating Scale (CMRS).11
For the DS, individualised domain scores go from 0 (normal) to 4 as follows: talking (incomprehensible), writing (unable to handle a pen), hygiene and dressing (requires 100% help), walking (wheelchair), and stair climbing (impossible). Eating is scored up to 3 (dependent on others). The total DS score range is 0−27.11
In the poster, validity and reliability of the CMRS was assessed.11 Study participants were adults with myotonia congenita (n=13) or paramyotonia congenita (n=12) from the randomised, crossover, double-blind mexiletine versus placebo MYOMEX trial.12 Two investigators used the CMRS to assess baseline scores at six centres in France.11 Vicart, who presented the poster, discussed during the poster question and answer session how the participants were all assessed in a temperature controlled room (20−25 oC), so that this would not be a variable. Interrater reliability was estimated by weighted κ coefficients (poor: <0.40; fair/good: ≥0.40–<0.75; excellent: ≥0.75).13 Intraclass correlation coefficients were calculated for global scores.14 Correlations with the visual analogue scale (VAS) stiffness score (primary efficacy criterion of the Myomex study) and with the Individualized Neuromuscular Quality of Life (INQoL) self-questionnaire15 were estimated using Spearman rank correlation coefficients.11
For the MSS, most κ coefficients for both frequency and severity showed ‘fair/good’ interrater reliability. Highest interrater agreement was in frequency of ‘eyelid blinking’ and severity of ‘respiratory muscle impairment’, which approached the cut-off for ‘excellent’ interrater reliability. Conversely, three domains were given ‘poor’ κ ratings for interrater agreement: severity of upper and lower limbs (right and left).11 For the DS, highest interrater agreement was observed for hygiene (rated as ‘excellent’), and dressing. However, eating and writing were in the ‘poor’ κ range. Overall, the intraclass correlation coefficients indicated moderate interrater reliability in CMRS severity and disability scores.11
The CMRS severity global score strongly correlated with both INQoL and VAS stiffness scores, both significantly. While the CMRS disability global score also strongly correlated with VAS, the correlation was not as strong for INQoL, although both were significant. The CMRS severity and disability global scores correlated well with each other, and
were significant.11
The study investigators concluded that the CMRS demonstrated moderate interrater reliability in this small exploratory analysis, and will continue to undergo validation in study populations with myotonic disorders.11 In two expert panel discussions, one in response to the IMPACT survey, unmet needs identified for patients with NDM included verified tools for diagnosis, and for monitoring the effectiveness of a drug treatment to manage myotonia symptoms over time.6,16 As such, the study investigators here concluded that the CMRS was “a promising scale for assessing the severity and impact of myotonia in patients with NDM.”11
Cardiac Assessment and Monitoring Recommendations for Patients with Non-dystrophic Myotonia Administered Mexiletine
Expression of both Nav1.4 sodium channel, and the CLC-1 chloride channel affected in NDM, is mostly confined to skeletal muscle, with an almost null expression in the heart.17,18 Accordingly, mutations in the SCN4A or CLCN1 genes in people with NDM would not be expected to affect cardiac function of patients with NDM, and indeed, cardiac impairment is not a symptom of NDM.19
Mexiletine, historically classified as a Class 1B antiarrhythmic, is a non-selective, voltage-gated sodium channel blocker that enhances sodium channel inactivation and repolarisation, and reduces skeletal muscle hyperexitability.20-22 It has a high affinity for Nav1.4 channels in the open state, and has no effect on potassium channels.21,22 Mexiletine is less potent, with quicker recovery from sodium channel binding than Class IA or IC sodium channel blockers.22 However, as mexiletine is also classified as an antiarrhythmic (indicated for treatment of documented, life-threatening ventricular arrhythmias),23 it is essential that a patient with NDM undergoes cardiac evaluation prior to and during mexiletine administration.24,25
Mexiletine has been used off label for myotonias for at least 40 years26 and, following a double-blind randomised trial (n=59),27 it is now the only anti-myotonic drug approved for NDM by the European Medicines Agency (EMA) and the Medicines and Healthcare products Regulatory Agency (MHRA) in the UK.1,25 Another double-blind randomised controlled trial,12 as well as analysis of Bayesian aggregated placebo-controlled n-of-1 trials of mexiletine,28 also showed the efficacy and safety of mexiletine in patients with NDM. These trials did not show any serious cardiac adverse events associated with mexiletine use in this patient population.12,27-29 In the first randomised controlled trial of mexiletine, one incidence of bradycardia was found at the end of Week 4 that resolved on follow-up without stopping treatment.27 In the second trial, and the n-of-1 trials study, no clinically relevant significant variations were observed in ECG readings at the end of the treatment period.12,28
In a long-term retrospective study based on data from a cohort of mexiletine-treated patients with NDM (n=59; treatment duration 1 month–20 years), no patients developed cardiac arrhythmias.30 In another study (n=63), ECG recordings compared with baseline found no significant changes in PR complex interval; heart rate; QRS duration; or in corrected QT interval when taking mexiletine. A total of 16 patients were referred to a cardiologist due to cardiac concerns prior to or during mexiletine administration but all were medically cleared to start or continue treatment.29 In a third study, 37 patients with NDM used an anti-myotonic medication, mostly mexiletine or ranolazine, with no reported cardiac adverse events.31
Despite these findings, an NDM expert panel discussed challenges around prescribing mexiletine included uncertainties about cardiac problems amongst their concerns.16 This may be due to mexiletine, and related antiarrhythmic drugs, having a U.S. Food and Drug Administration (FDA) Black Box warning following findings in an encainide or flecainide study, where excess mortality or non-fatal cardiac arrest occurred in patients with recent myocardial infarction.32 However, mexiletine itself was not tested in this trial, and this warning is not in the mexiletine licensed to treat myotonia in people with NDM summary of product characteristics.25
With cardiac concerns in mind, a treatment algorithm was developed to aid prescribers, as described in a poster by Vicart and colleagues, presented by the lead author at the EAN 2023 congress.33 To help define the treatment algorithm, three workshops brought together three French neurologists and five French cardiologists. They utilised their expertise, alongside the mexiletine summary of product characteristics25 and a review of the literature regarding mexiletine safety,12,22,27,28-31 to construct an algorithm to define the screening and surveillance tools needed to help avoid cardiac events in patients treated with mexiletine.33
Components of the Algorithm
Stage 1: Assessment
Prior to mexiletine initiation, the patient should be evaluated by a cardiologist, to screen for cardiac contraindications based on medical history, and systematic ECG and echocardiography investigations.33 According to the summary of product characteristics (mexiletine indicated in adult NDM), medical history that precludes the use of mexiletine includes myocardial infarction; atrial fibrillation or atrial flutter; angina or non-revascularised coronary artery disease; ventricular tachycardia; complete heart block (i.e., third-degree atrioventricular block) or heart block that may evolve to complete heart block; and cardiac disease-modifying therapy and drugs that may cause torsades de pointes, including ajmaline, amiodarone, disopyramide, dofetilide, dronedarone, encainide, flecainide, ibutilide, moricizine, procainamide, propafenone, quinidine, sotalol, and vernakalant.25
Systematic investigations also need to be carried out. For ECG, findings that rule out mexiletine use include bundle branch block; wide QRS complex (≥120 ms); sinus node dysfunction (heart rate <50 beats per min); bifascicular or trifascicular block; high-degree atrioventricular block (Mobitz II or complete block); first-degree atrioventricular block with PR duration of ≥240 ms; and necrosis Q wave and repolarisation abnormalities.25
Findings on echocardiography that preclude mexiletine use include segmental wall motion abnormality and a left ventricular ejection fraction below 50%.25
Stage 2: Monitoring 33
Mexiletine can be introduced if the above are ruled out. The maximum effective dose is usually reached 3 weeks after the first dose, when a follow-up ECG should be carried out. A cardiologist’s opinion is needed if changes are observed, or if a patient develops any new cardiac symptoms, such as syncope, chest pain, or unusual palpitations.33
Once mexiletine is initiated, an ECG should be carried out at least every 2 years, a systematic cardiology consultation should be carried out every 5 years, including screening for coronary artery disease and a cardiovascular risk assessment.33 For patients with a known cardiac abnormality detected prior to treatment but which not contra-indicated mexiletine use, it is recommended to perform a cardiac evaluation every year, or more frequently if necessary.25
During presentation of the poster, Vicart concluded by saying that the authors hope the algorithm will “assist the team caring for patients with NDM, and will enable accurate screening and monitoring to avoid cardiac events during treatment.”33
Conclusion
Care of patients with NDM requires assessment of myotonia severity and impact and should include symptom monitoring both prior to and during medication prescription, if needed. The CMRS may provide a validated tool by which myotonia can be easily, systematically, and consistently assessed.11 For patients with NDM treated with mexiletine, an algorithm for cardiac safety monitoring has been developed to assist neurologists and cardiologists managing these patients.33







