Meeting Summary
Vyepti▼(eptinezumab) is a monoclonal antibody (mAb) targeted against calcitonin gene-related peptide (CGRP), which is indicated for the prophylaxis of migraine in adults who have at least four migraine days per month. During this symposium, a panel of leading headache experts discussed the potential for an intravenous (IV) anti-CGRP therapy to advance current migraine management and elevate overall treatment expectations. Brendan Davies, Consultant Neurologist at University Hospital of North Midlands, UK, and Chairman of the British Association for the Study of Headache, UK, highlighted the substantial burden imposed by migraine and explored the potential for improved pharmacological delivery to enhance persistence, which is key to long-term therapeutic efficacy. Andrew Blumenfeld, Director of the Los Angeles Headache Center, California, USA, explained how the features of immediate drug delivery and sustained duration of action were taken into consideration when developing eptinezumab, and outlined evidence from the three pivotal Phase III clinical trials supporting its efficacy and tolerability as a migraine preventive therapy. Finally, Manjit Matharu, Professor of Neurology at University College London, UK, shared real-world experience on the application of eptinezumab in clinical practice and provided insights into identifying patients who may benefit from IV anti-CGRP therapy.
Should Patients Expect More From Their Migraine Therapy?
Brendan Davies
Davies began by highlighting the substantial burden that migraine imposes on patients, the workforce, and primary and secondary care. Data from the UK reveal that nearly one-third of migraine sufferers have moved from full-time to part-time work due to the condition, and a quarter have left their job entirely. In 2021/2022, there were at least 33,000 hospital admissions for migraine in England, an increase of 31% compared to the previous 5 years. Unsurprisingly, therefore, the Migraine Trust has declared that migraine care in the UK is “heading in the wrong direction”.1
Despite the impact of migraine on healthcare systems, awareness and training on migraine recognition and management remains lacking.2 Migraine is not currently a core part of medical student, junior doctor, or general practitioner (GP) training in the UK, and there is no universally used primary care tool for migraine assessment across health economies. Despite their proven efficacy, major variation also exists in the ease of accessing targeted therapies for migraine across not only the UK, but also Europe and the wider world. Furthermore, there is no public health strategy or UK health board national strategy for delivery of these migraine therapies.1 Patients with migraine in the UK should expect better, Davies acknowledged.
US data from the pre-CGRP era highlight the substantial challenges of real-world effectiveness and adherence in chronic migraine. In this 4-year retrospective claims analysis, which included around 8,700 patients, drug adherence was found to be only 26−29% at 6 months, dropping to 17−20% after 1 year.3 Persistence on first drug treatment was 24−26% at 6 months and 13−16% at 12 months.4 Approximately half of patients discontinued therapy in less than 60 days and, for those that stopped and then reinitiated their first treatment, only 4–8% persisted at 12 months.4 Equally poor persistence was observed after switching. Nearly one-quarter of patients switched to another treatment following their first or second therapy, but only 10–13% persisted with the new treatment at 12 months.4 Suboptimal adherence and persistence have therefore adversely impacted on the real-world effectiveness of these migraine treatments, Davies concluded.
Against this backdrop, Davies highlighted important opportunities that exist to reduce the impact of migraine moving forward by improving the pharmacological delivery of therapy. Appropriately targeted treatment options, relevant to the pathophysiological processes that cause migraine, are important, he emphasised. There is also a need for improved bioavailability and different options for drug administration, so as to provide the opportunity for expedited onset of action. Efficacy and tolerability are both important to support adherence over time, and therapeutic effectiveness should be sustained for the long-term. Ultimately, patients want to achieve freedom from their migraine, Davies asserted. Other important considerations include safety and convenience, coupled with accessibility to individuals and within systems. Patients with migraine need access to diagnosis, treatment, and knowledgeable healthcare professionals (HCP), he concluded.
Why an IV Anti-CGRP Therapy Should Be Considered
Andrew Blumenfeld
Migraine exerts a profound effect on patients’ lives.5 As Blumenfeld reported from his clinical practice, in addition to headache severity and disability, patients can feel imprisoned by their migraines, with the unpredictability of attacks and associated anxiety generating a substantial interictal burden. Data from the International Burden of Migraine Study, an internet-based survey of 8,726 patients, revealed a clear link between headache frequency and increased levels of disability, as measured by the Migraine Disability Assessment (MIDAS) score.5 Disability increases as patients move from episodic to chronic migraine, he explained, with an ‘inflection point’ at around 11−12 days where disability escalates substantially.5
Blumenfeld confessed that, in the previous era of non-specific migraine therapy, many HCPs, like their patients, felt “imprisoned” by the limited treatment choices available. However, the evolution of CGRP inhibitors since 2018 has helped to improve outcomes for migraine patients. One of these agents is eptinezumab, a CGRP-targeted mAb approved for the prophylaxis of migraine in adults who have at least 4 migraine days per month.6
Eptinezumab is an IgG antibody that binds CGRP in a deep pocket between the light and heavy chains.7 All six complementarity-determining regions of eptinezumab make multiple contacts with CGRP, enabling high-affinity binding with a slow dissociation rate.7 “It’s the CGRP that binds to receptors and triggers a migraine attack, so you want to have a drug that is going to bind CGRP as tightly as possible,” Blumenfeld remarked.7
Eptinezumab binds selectively to the CGRP ligand, thereby reducing off-target activity and ensuring a sustained duration of action.7-10 “Eptinezumab is 100% bioavailable in the circulation post-infusion, so can start binding immediately to CGRP,” Blumenfeld explained.7 Pharmacokinetic data for eptinezumab obtained in early phase studies support lower dose levels and a longer administration schedule for IV versus subcutaneous (SC) dosing.10,11 Time to peak plasma concentration is reached within the first few hours with IV dosing of 100 mg eptinezumab, while 100 mg administered SC takes around a week to reach equivalent levels.11 Both then maintain therapeutically active levels for about 12 weeks.11 The time required for absorption of SC administration may therefore lead to loss of active drug or reduced bioavailability, Blumenfeld pointed out.11 However, it is important to note that the SC formulation of eptinezumab is not approved for the treatment of migraine, and the relationship between the pharmacokinetic profile of the two routes of administration and the clinical properties have not been studied.
Three pivotal clinical trials have helped to establish the efficacy profile of eptinezumab in migraine prevention. PROMISE-1 and PROMISE-2 were Phase III, multicentre, randomised, double-blind, placebo-controlled, parallel-group studies.12,13 In PROMISE-1, 898 adults (18−75 years of age) with episodic migraine were randomised to eptinezumab 30 mg (NB: A 30 mg arm was included in this registration trial but is not a licensed dose), 100 mg, 300 mg, or placebo for up to four IV doses administered every 12 weeks.12 PROMISE-2 enrolled 1,121 adults (18−65 years of age) with chronic migraine who were randomly assigned to receive eptinezumab 100 mg, eptinezumab 300 mg, or placebo administered on Day 0 and Week 12.13 DELIVER was a multicentre, multi-arm, Phase IIIb trial comprising a 24-week double-blind, placebo-controlled period and a 48-week dose-blinded extension. In this trial, 892 adults (18−75 years of age) with episodic or chronic migraine who had failed on two-to-four preventative medications were randomly assigned to treatment.1 The primary endpoint for all studies was change from baseline in mean monthly migraine days (MMD) over weeks 1–12; key secondary outcomes included ≥75% and ≥50% migraine responder rates.12-14
Efficacy results from all three clinical trials were positive, with eptinezumab significantly reducing the number of MMDs versus placebo at both 100 and 300 mg doses (Figure 1).12-16
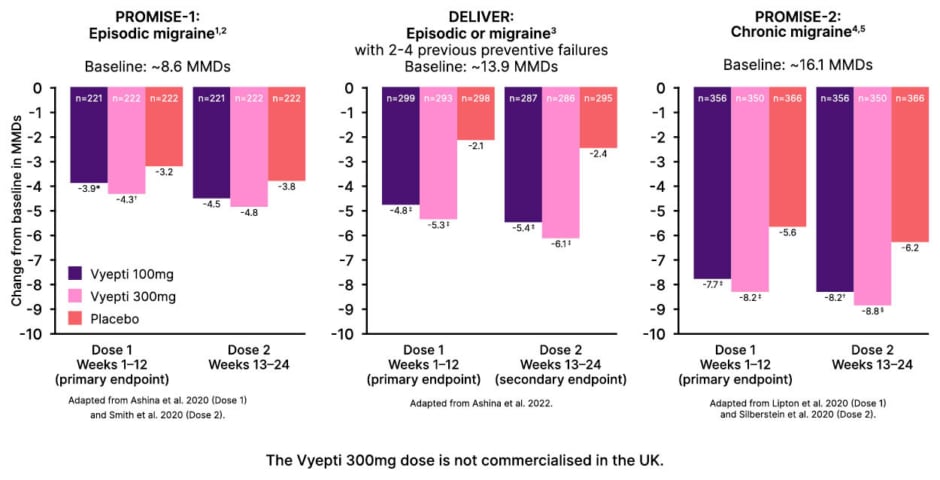
Figure 1: Observed efficacy results of eptinezumab versus placebo in pivotal clinical trials.12-16
*p=0.0182
?p=0.0001
‡p<0.0001
§nominal p<0.001 versus placebo.
MMD: monthly migraine day.
In terms of secondary endpoints, eptinezumab administration led to a significant increase in the number of patients experiencing ≥75% migraine response within the first month from baseline in both the PROMISE-1 and PROMISE-2 studies.12,13,15,16 Blumenfeld described it as “interesting” that the proportion of ≥75% responders increased to ~40% after the second dose of eptinezumab (compared to around 24% in the placebo groups).12,15 He therefore cautioned against labelling patients as non-responders after only one eptinezumab treatment cycle (however, he also acknowledged the challenges faced with respect of NICE guidance stopping criteria in the UK).17
In the DELIVER trial in patients who had failed on two-to-four prior oral preventives, eptinezumab reduced mean monthly migraine attacks and also decreased the severity of remaining migraines versus placebo (Figure 2).14 This reduction in headache intensity should correlate to an improvement in overall function, Blumenfeld remarked. Indeed, across all treatment groups in DELIVER, Headache Impact (HIT-6) scores had significantly improved by Week 12 with eptinezumab versus placebo.14 Eptinezumab also led to significant improvements in patients’ perceived impact of migraine (as Patient Global Impression of Change) score, with 59% of patients in the active treatment groups reporting ‘much improved’ or ‘very much improved’ symptoms after just 1 month of treatment, compared with 20% in the placebo group.18 In terms of onset of action, Blumenfeld explained that benefit could be seen with eptinezumab from as early as Day 1 after infusion. Analysis of data from the PROMISE-1 and PROMISE-2 trials to determine the onset of preventive efficacy with eptinezumab showed ~50% reduction in patients’ likelihood of experiencing a migraine on Day 1, compared to ~25% with placebo (Figure 3).9
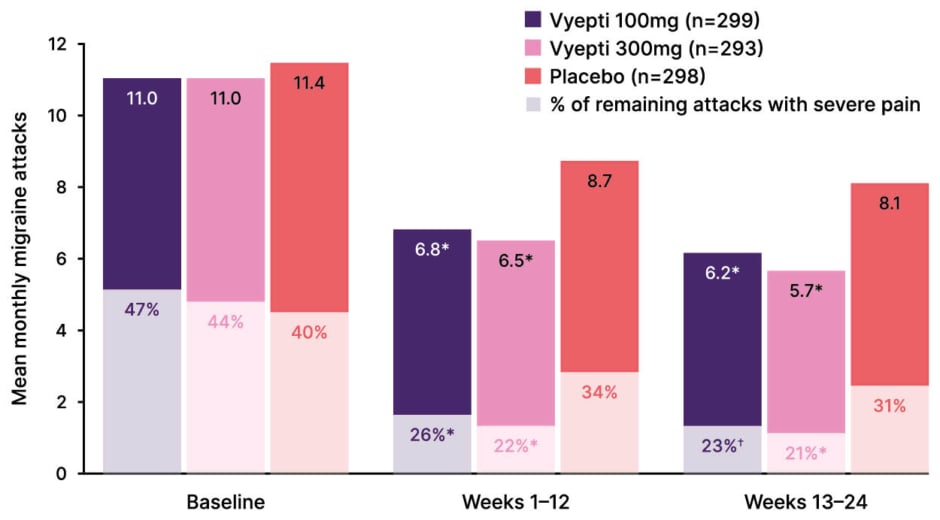
Figure 2: Mean monthly migraine attacks and percentage of migraine attacks with severe pain intensity.14
*p<0.0001 versus placebo
?p=0.0001
Data represent a combined analysis of a secondary (percent of migraine attacks with severe pain intensity in Week 1-12) and exploratory endpoint (percent of migraine attacks with severe pain intensity in Week 13-24), P-values are comparisons with placebo, and as secondary/exploratory outcomes, are not controlled for multiplicity.
Adapted from Ashina et al.14 2022 (supplementary appendix).
When patients in the DELIVER study were followed out to 72 weeks, the proportion achieving ≥50% reduction in MMDs was maintained over the long-term. Furthermore, patients in the placebo group who switched to eptinezumab in the open-label extension showed similar sustained treatment responses as those who started in the eptinezumab group from study outset.19 In the 2-year PREVAIL study, an open-label, Phase III trial evaluating the long-term safety and tolerability of eptinezumab in chronic migraine, the majority of treated patients reported little or no disability, or mild disability, at Weeks 12–104, compared to baseline.20
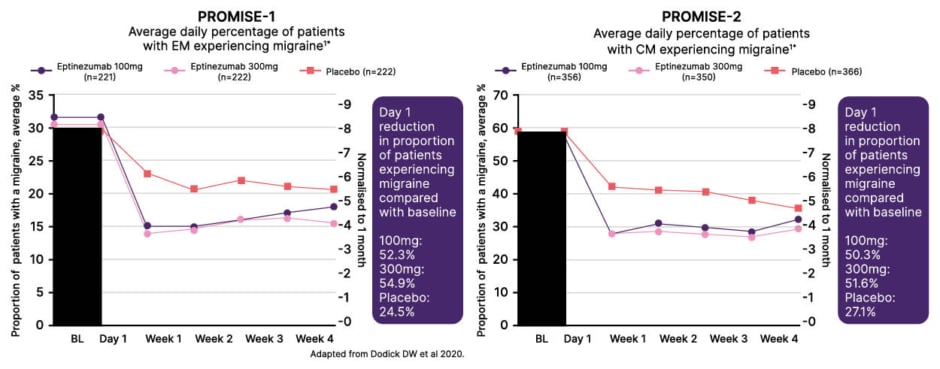
Figure 3: Onset of preventive efficacy with eptinezumab.9
The Vyepti 300mg dose is not commercialised in the UK.
Adapted from Dodick DW et al.9 2020.
BL: baseline.
In terms of safety profile, eptinezumab is generally well tolerated in patients with migraine, with most adverse events proving transient and mild to moderate in severity.6,14,19,20-22 The most common treatment-emergent adverse events (TEAE) reported in a pooled safety analysis of 701 patients treated with 100 mg eptinezumab (versus 791 on placebo) were nasopharyngitis (6.3% versus 5.2%), upper respiratory tract infection (6.4% versus 6.1%), fatigue (2.9% versus 1.6%), and dizziness (3.9% versus 2.7%).21 Serious hypersensitivity reactions, including anaphylactic reactions, have been reported with eptinezumab and may develop within minutes of infusion.6 Hypersensitivity events typically resolve within 1 day and the incidence of all side effects generally decreases after subsequent doses of eptinezumab.21 Development of anti-drug antibodies and neutralising antibodies has not been shown to impact the efficacy and safety profiles of eptinezumab.23 In terms of cardiovascular (CV) safety, eptinezumab demonstrated a low overall incidence of CV-related TEAEs, with rates similar to placebo, and no clinically relevant changes in vital signs.24 Importantly, no new safety signals were observed in DELIVER or in the DELIVER extension study with long-term treatment.14,19
Blumenfeld noted that the main side effects seen with eptinezumab are not those commonly associated with anti-CGRP drugs as a class, such as constipation, hypertension, and skin reactions.21 What is observed instead are non-allergic nasopharyngeal symptoms, typically nasal congestion and sneezing.21 From a pathophysiological point of view, these side effects occur because blockade of the CGRP-driven pathway leads to compensatory activation of the parasympathetic system, which supplies the nasal mucosa.25
In the clinic setting, treatment with eptinezumab involves a 30-minute infusion administered by an HCP every 12 weeks.6 The recommended dosage is 100 mg, and no loading dose is required. Blumenfeld described the rate of discontinuations with eptinezumab as “low”, with less than 2% of patients stopping treatment due to adverse effects.21 However, the treating HCP should observe or monitor patients during and after the infusion in accordance with normal clinical practice.6
Finally, Blumenfeld briefly reviewed a clinical case from his own practice in the US showing successful real-world treatment outcomes with eptinezumab treatment in a needle-phobic patient with high-frequency episodic migraine. In this patient, MMDs were reduced from 8−14 at baseline to 2−3 after two infusion cycles.
Blumenfeld concluded his presentation by summarising the body of evidence from clinical trials, in which eptinezumab demonstrated significant reductions in the number of MMDs in patients with chronic or episodic migraine in Weeks 1–12 compared with placebo, whilst also reducing mean monthly migraine attacks and the severity of remaining migraines.12-14 The majority of treated patients with chronic migraine reported little or no disability at Weeks 12–104 versus MIDAS total score at baseline.20 In terms of safety, eptinezumab proved generally well tolerated, with less than 2% of patients discontinuing treatment due to adverse effects, based on the pooled analysis.21 Importantly, the drug also started working from Day 1 after IV infusion compared to baseline.9,12,13 As the only intravenous anti-CGRP mAb currently available, eptinezumab may therefore have the potential to raise treatment expectations in migraine, Blumenfeld concluded.
A Practical Insight Into Managing Patients
Manjit Matharu
Matharu began with an overview of the current armamentarium of migraine treatments, summarising their evidence base and guidelines positioning. “It is within this landscape that we have had the introduction of eptinezumab in the UK,” he noted. Non-specific preventive treatments include tricyclic antidepressants, beta blockers, anticonvulsants, calcium channel antagonists, angiotensin II receptor blockers, and nutraceuticals.26-29 However, many of these agents are not licensed (including calcium channel antagonists, angiotensin II receptor blockers, and nutraceuticals) specifically for migraine in the UK and carry warnings and precautions, Matharu pointed out, notably the MHRA updates related to restrictions on use of topiramate and sodium valproate due to teratogenicity concerns.30,31
Specific and emerging preventive treatments for migraine include botulinum neurotoxin, in the form of onabotulinumtoxinA; CGRP mAbs, which include eptinezumab, along with erenumab, fremanezumab, and galcanezumab; and the oral CGRP receptor antagonists rimegepant and atogepant.32-34 Matharu outlined NICE conditions for use of these agents according to their technology appraisal guidelines and the stopping criteria, describing these as “barriers to use, which have been created on the basis of cost”. For both CGRP mAbs and CGRP receptor antagonists, NICE stipulates that therapy must be stopped after 12 weeks if the frequency of episodic migraine does not reduce by at least 50% or chronic migraine by at least 30%.17,35-39
Matharu then went on to present his own clinical practice experience with eptinezumab in migraine management at the National Hospital for Neurology and Neurosurgery. This clinical evaluation has been conducted by Matharu and reflects clinician’s own clinical experience and data. This prospective, clinical evaluation of eptinezumab’s real-world safety and efficacy included a total of 48 adult patients (≥18 years old; 73% female), 41 with chronic and seven with episodic migraine. The primary outcome assessed was reduction in mean monthly headache days at 12 weeks. Matharu emphasised that patients in this study had failed on an average of six prior preventative therapies; virtually all patients (96%) had been treated with prior botulinum neurotoxin injections, and 42% had previously tried SC CGRP mAbs.
In this cohort of patients that informed the clinical evaluation, eptinezumab proved effective, with patients achieving a 9.2 (±7.9) day reduction in monthly headache days, from 23.3 (±6.6) at baseline to 14.1 (±9.0) at 12 weeks. In terms of response categories, 16% of patients had an ‘excellent’ response (80–100% reduction in patient-reported monthly headache days), 31% ‘good’ (50–79% reduction), 20% ‘partial’ (30–49% reduction), 31% ‘mild/none’ (0–29% reduction), and 2% ‘worsening’. Eptinezumab administration also led to notable improvement in key secondary outcomes, including responder rates, disability scores, and affective scores for anxiety and depression at 12 weeks.
Overall, 63% of patients with chronic migraine and 71% with episodic migraine showed ≥30% and ≥50% improvement, respectively. Disability was reduced by 25.7 points from baseline on the MIDAS score and by 6.8 points on HIT-6 at 12 weeks. A positive response to eptinezumab was observed in 45% of patients who had failed to respond to a SC CGRP mAb. Importantly, three out of 15 patients receiving regular botulinum neurotoxin injections were able to stop these once on treatment with eptinezumab. Safety data from this clinical evaluation showed that four out of 48 patients treated with eptinezumab experienced an adverse event, all of which were mild in nature, and no patients discontinued treatment as a result. Two patients reported flu-like symptoms, two had mild headache worsening, and one had gastrointestinal upset.
Summarising the key observations from this real-world dataset, Matharu concluded that eptinezumab is effective as a preventive treatment in a relatively refractory migraine cohort, the majority of whom had previously failed to respond significantly to botulinum neurotoxin injections. Efficacy was observed within both botulinum neurotoxin and CGRP mAb non-responders. Treatment with eptinezumab produced a substantial reduction in headache days and disability scores and was generally well tolerated, with mild and manageable side effects and no discontinuations due to adverse events. However, Matharu acknowledged that this evidence is from a relatively small cohort at one tertiary referral centre, and therefore further real-world data is still required.
Based on his own clinical experience, Matharu went on to highlight certain patients who may be particularly suitable for this treatment approach. Eptinezumab is appropriate for both episodic and chronic migraine occurring on at least 4 days per month, and has shown efficacy in patients with coexisting medication overuse headache.40,41 It may also be a useful option for patients who have not responded well to other preventives, including oral medications or injectables such as botulinum neurotoxin (as efficacy has been demonstrated in previous treatment failures) and/or for patients who have experienced side effects or poor tolerability with oral migraine preventive treatments.
Eptinezumab is well suited to patients who prefer an infrequent dosing regimen (every 12 weeks), which, Matharu suggested, may also improve compliance. For patients who have difficulty with self-administering injections, and who may experience anxiety, discomfort, or practical issues, infusion overseen by a HCP may be more appealing. Based on his own experience, Matharu noted that patients with needle tolerance are generally comfortable with eptinezumab administered via intravenous infusion. Speed of onset is also an important consideration with eptinezumab, which has an onset of action within 24 hours due to its administration route.12,13
Matharu also flagged certain types of patients who may be less suitable for treatment with eptinezumab. This includes women who are pregnant or breastfeeding, patients with needle phobia, and those with comorbid conditions, notably CV disease or Raynaud’s syndrome.6,17,42 Eptinezumab is contraindicated in individuals with a hypersensitivity to the active substance and the excipients, as per the Summary of Product Characteristics (SmPC).6 Although patients with limited access to healthcare facilities for regular infusions may find it challenging to initiate/maintain the treatment regimen, Matharu stressed that “this access issue is something that we can address”.
In contrast to most HCPs’ expectations, a recent patient preference study showed that IV dosing is actually viewed quite favourably by patients with migraine. In a US study of 604 adults with diagnosed migraine, a significant proportion said they were either indifferent with regard to IV injections and self-injections or had a strong preference for IV infusions administered by HCPs (28%). Only 21.2% preferred self-injection.43 Key factors influencing these preferences were healthcare provider involvement and speed of onset. Interestingly, 25.5% of patients indicated that rapid onset was a preferred attribute in their treatment choice.43
Summing up, Matharu offered his own clinical perspective on why eptinezumab should be available for patients with migraine. First, offering IV options helps to address diverse patient needs and preferences, as not all patients can benefit from self-injections or oral treatments. Many patients also prefer infusion treatments due to the reassurance of HCP involvement. This clinical evaluation supports the observations from the clinical trial programme that eptinezumab is effective and has a generally well tolerated safety profile. Furthermore, Matharu stressed the onset of action within 24 hours and why it is important for patients requiring timely symptomatic improvement.12,13 Finally, there is the issue of treatment accessibility and choice; ensuring the availability of eptinezumab will allow clinicians to offer the most appropriate treatment for each patient’s unique needs, he concluded.







