Abstract
Mechanical insufflation-exsufflation (MI-E) is an intervention used for cough augmentation in patients with neurological conditions with bulbar impairment. This study aimed to explore implications for clinical practice in the use of transnasal laryngoscopy during MI-E.
Twenty-one patients underwent MI-E application with simultaneous transnasal laryngoscopy. Pressures were commenced at +15 cm H2O/-15 cm H2O and titrated according to an agreed algorithm. Outcomes collected included baseline bulbar function scores, optimal MI-E settings, and/or alternative secretion management strategies including medication recommendations.
Changes to pressure and/or modality were implemented in all but one patient. No adverse effects of laryngoscopy were seen. For patients with progressive bulbar palsy, limb onset amyotrophic lateral sclerosis, and other conditions with bulbar impairment, transnasal laryngoscopy resulted in discontinuation of MI-E in 54%, 0%, and 14%, respectively. Pressure changes were made for all patients remaining on MI-E and medication changes were made for 47% of patients across all conditions.
Transnasal laryngoscopy can be utilised to safely assess the impact of MI-E on laryngeal structures to optimise settings for patients with bulbar impairment. Cough augmentation strategies can be tailored accurately for patients with a variety of neurological conditions with bulbar impairment based on the results of transnasal laryngoscopy.
Key Points
1. Mechanical insufflation-exsufflation (MI-E) is a commonly used tool for cough augmentation and secretion clearance in patients with neurological conditions. However, methods for ongoing efficacy review are lacking in accuracy.2. This is a retrospective observational study showing the safety and feasibility of carrying out transnasal laryngoscopy during the use of MI-E.
3. Individualisation of MI-E settings through the simultaneous use of transnasal laryngoscopy is a safe way of reviewing and in some cases extending, its effective use in patients with neurological disorders and bulbar impairment.
INTRODUCTION
Individuals with neuromuscular diseases (NMD), where deterioration in bulbar function coexists with respiratory muscle weakness, are at risk of dysarthria, dysphagia, and impaired cough. This contributes to difficulties in clearing oropharyngeal secretions, choking, laryngospasm, and aspiration.1,2 Bulbar impairment is caused by injury or degeneration of the corticobulbar tract and results in weakness or abnormalities in the control of hypopharyngeal and laryngeal structures.3 Pseudobulbar palsy can be a presenting symptom in a number of conditions, including bilateral cerebral infarction, demyelinating motor neuron diseases, tumours of the brain stem, amyotrophic lateral sclerosis (ALS), Parkinson’s disease, multiple sclerosis, and trauma.4
For patients with dysphagia, with or without vocal fold motion impairment, alongside respiratory muscle weakness, the consequences are two-fold: the inability to protect the airway during swallowing, resulting in aspiration, and the inability to generate subglottic pressure for production of an effective cough.3 This combination gives rise to a significantly greater susceptibility to respiratory infection, hospitalisation, and in some conditions, long-term ventilation.5-10 In addition to the risk of infection, malnutrition secondary to dysphagia has been shown to be a significant co-existing condition linked with poor survival rates and worsening respiratory function.11
Chest clearance techniques provide a proactive and preventive approach to the symptomatic relief and management of secretions for patients who have neurological conditions with bulbar impairment. Simple airway clearance techniques (ACT) require a level of respiratory muscle strength that renders them unfeasible as neurological status deteriorates. Mechanical insufflation-exsufflation (MI-E) requires reduced physical input and offers several modes of delivery to allow for individualised patient care. Positive and negative pressure is sequentially applied to the airways to increase the expiratory flow rate and peak cough flow (PCF), facilitating the movement of pulmonary secretions. There have been a number of studies that have further examined the mode and benefits of action validating its use in neurological conditions.9,12-19 In addition to a reduced physical burden, MI-E has been shown to generate higher PCF values than other methods of cough augmentation in patients with NMD and other conditions, and as such,15,19-21 has been cited as the most effective treatment in patients with respiratory muscle weakness secondary to NMD.22,23
Despite compelling evidence for its use when simple ACTs are not effective, MI-E can also become challenging as neurology deteriorates further. This can in turn be attributed to advancing bulbar impairment, with potential for vocal fold abduction, motion impairment (inability to maintain open vocal folds), and an exaggerated response to stimuli.24
There are no standard guidelines for the set-up or progression of MI-E; however, high pressures have long been considered the most effective at secretion clearance, and the inclusion of an expiratory flow bias is proposed as a crucial component.16,25 More recently, MI-E during laryngoscopy has been shown to provoke varying levels of laryngeal response in healthy individuals using pressures ranging from +/-20 to +/-50 cm H2O.26 A few studies in individuals with ALS showed treatment failure most likely during the insufflation phase, due to laryngeal adduction, with high pressures being most likely to provoke an adverse laryngeal response.27,28
This study considers further clinical findings and implications of using transnasal laryngoscopy when applying MI-E in patients who have neurological conditions with bulbar impairment, and whether direct visualisation can contribute heavily towards optimum efficacy and individualisation of therapy.
Objectives
The primary aim was to determine the most efficacious cough augmentation strategy whilst determining whether simultaneous MI-E and visualisation is a clinically viable, effective technique to achieve this.
MATERIALS AND METHODS
Design and Setting
The team conducted a retrospective observational study using analysis of routinely collected data from 21 patients referred to a joint physiotherapy/speech and language therapy (SLT) clinic within the Cough Augmentation Service.
At the tertiary care centre, patients with neurological conditions alongside bulbar impairment were routinely assessed for the provision of an MI-E device. Suitability was based upon bedside subjective and objective assessment with reference to contraindications and precautions discussed by Swingwood et al.29 and listed in the instructions for use of the Clearway 2 (Breas Medical, Mölnlycke, Sweden) and E70 (Philips Respironics, Murrysville, Pennsylvania, USA) cough assist devices.30,31
With a new awareness of laryngeal response to MI-E and an increasing number of patients with neurological conditions and bulbar impairment reporting a change in physical sensation whilst using MI-E or a feeling of reduced efficacy, a joint clinic was set up for visualisation of the larynx during MI-E. Visualisation via transnasal laryngoscopy was carried out based on the work of Andersen et al.26-28 The clinic was run jointly by a specialist SLT and a specialist respiratory physiotherapist. All referred individuals were triaged for suitability for the clinic, and consented prior to the visualisation and assessment procedure.
The Health Research Authority research ethics decision tool (National Health Service [NHS], London, UK) confirmed that this retrospective observational analysis of routinely collected clinical data in this patient cohort did not require formal research ethics committee approval.
Inclusion and Exclusion Criteria
Inclusion criteria included adults with neurological conditions alongside bulbar impairment within the service, who were already established onto MI-E or had been referred for set-up with concerns regarding tolerance of effective MI-E. As this group of patients had already been risk assessed for use of MI-E, there were no further criteria to screen against for exclusion, and no patient was excluded due to learning difficulties, severe bulbar impairment, or requirement of nasopharyngeal or oropharyngeal suction. Patients with suspected laryngospasm episodes were monitored closely with adrenaline nebulisers, heliox, and medical support all readily available, if required, during the procedure.
Subjects
Assisted cough techniques were considered for patients with a PCF of 270 Lpm or less, and for patients with NMD with a PCF above 270 Lpm with a history of recurrent chest infection.32
Patients were referred into the joint SLT/physiotherapy clinic if they reported or were observed to display the following changes in response to current MI-E regimes: reduced synchronicity with MI-E, incomplete chest expansion on insufflation (often with associated cheek/throat bulging), reports of being ‘unable to breathe’ during insufflation and/or exsufflation, reduced secretion clearance, and/or recurrent chest infection despite continued compliance with MI-E. Standard review methods were trialled at bedside in the first instance prior to referral into the clinic. These included alterations to timings, pressure, flow, and treatment regimes.
Severity of Bulbar Dysfunction
Bulbar impairment was recorded according to swallowing status using the International Dysphagia Diet Standardisation Initiative Functional Diet Scale (IDDSI-FDS), as provided by their local SLT.33 The lower the score, the higher the degree of diet or fluid modifications required.
Speech functional status was scored by SLT using the ALS Severity Score (ALSSS) speech subscore during the clinic appointment.34 The higher the score, the higher the degree of impairment.
Video-Recorded Transnasal Fibre-Optic Laryngoscopy During MI-E
Using DiVAS software (DP Medical Systems, Chessington, UK), transnasal fibre-optic laryngoscopy (XION Medical EndoFLEX System, Berlin, Germany) with video nasopharyngoscope (EV-NC tube diameter 3.5 mm) was used to visualise hypopharyngeal and laryngeal structures at baseline and response patterns during MI-E application using the E70 Cough Assist device set according to a standardised MI-E protocol. An appropriately sized Quadralite anaesthetic face mask (Intersurgical Complete Respiratory Systems, Berkshire, UK) was adapted with a hole punched in it to pass the scope. An attempt would be made to occlude the hole with the fingers once the scope was in position. The authors acknowledge this method does not fully negate air leak around the scope; however, subjective detection of air leak was considered insignificant.
Baseline assessment of hypopharyngeal and laryngeal structures commented on any changes in structure or movement. Observation of location, colour, and viscosity of secretions were made and were used in conjunction with a validated secretion scale (New Zealand Secretion Severity Score [NZSS]) by a flexible endoscopic evaluation trained SLT.35 Any concerns with high or tenacious secretion load were discussed with the team’s laryngology or respiratory physician colleagues.
No local anaesthetic was used during this procedure as per national guidelines, and because use of lidocaine may impact the laryngeal adductor reflex, which would interfere with the assessment of laryngopharyngeal sensory impact with MI-E.36,37 At times, flexible endoscopic evaluation with oral trials was clinically indicated for some patients as a part of standard dysphagia care, and was performed pre- or post-MI-E assessment during the same procedure.
MI-E Protocol and Target Outcome
Airway patency alongside patient feedback were the main outcome measures for pressure tolerance trials, with settings at this point considered to be optimal for the individual.
A standardised MI-E protocol was used: initial pressures started at +15/-15 cm H2O for a duration of 1.8/1.8 seconds each, low flow, no oscillations. Pressures were increased by increments of 2–5 cm H2O. Pressure increases were determined by patient feedback and real-time assessment of the impact on hypopharyngeal and laryngeal structures. Pressures were increased for as long as the airway remained patent (vocal folds and subglottis still visualised) and the patient was compliant. If airway patency was not visualised at the lowest pressure settings applied, alternative techniques such as breath stacking were taught using visual feedback via the monitor and biofeedback via placement of physiotherapist’s hand on the patient’s chest. Figure 1 illustrates the steps used to troubleshoot airway shutdown and pressure settings to determine optimal settings.
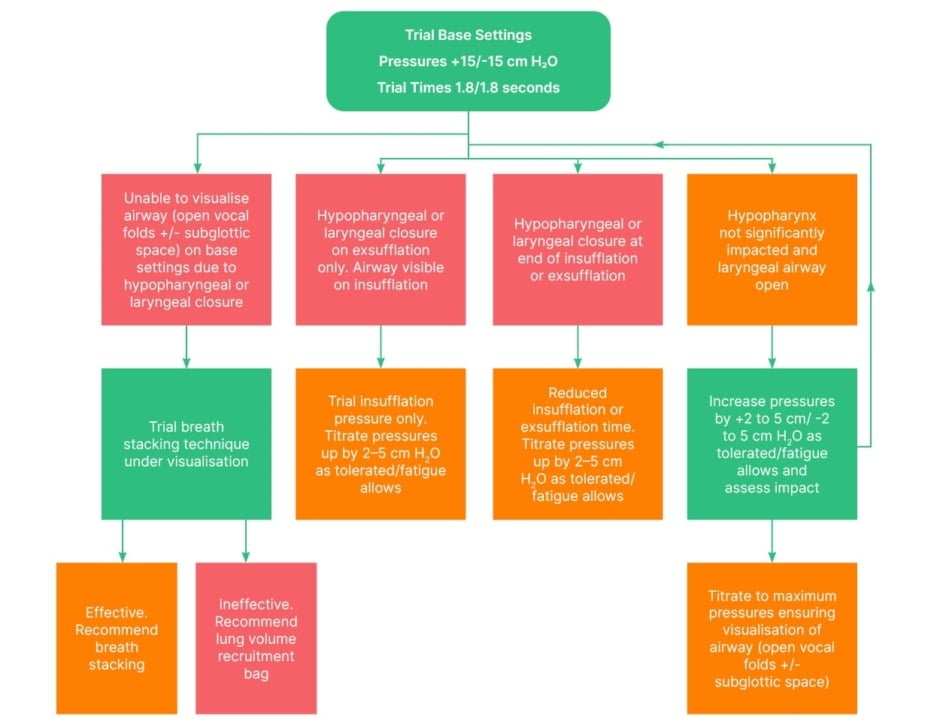
Figure 1: Proposed flow chart to guide trials.
Images were captured, reported, and archived on the hospital electronic patient record, then interpreted and reported at the bedside utilising validated scoring systems evaluating hypopharyngeal and laryngeal anatomy for signs of secretions, physiology, and function.38 Each patient had an individualised report documenting structural changes, quantified secretion load, and the impact of any modifications to pressure, time, or flow rate on the laryngeal and pharyngeal structures. Any structural abnormalities seen were referred for review by the Ear, Nose, and Throat team.
Post-procedural Recommendations
Recommendations for medication changes were made based on observations of secretion load, viscosity, current medication regime, and likely origin of secretions based on colour and location of pooling (salivary, nasal, pulmonary). Suggestions followed national guidance for the management of hypersalivation and were reviewed by the medical team or general practicioner.39
Data Analysis
Retrospective tabulation of data was completed from clinician documentation. Data collection included participant demographics (age, medical diagnosis, sex), baseline swallowing and cough augmentation (IDDSI-FDS and ALSSS), baseline cough augmentation recommendations (MI-E settings if applicable or other ACTs), reported frequency of chest infections in the preceding 12 months, and reason for referral into the clinic. Data were also collected on the optimal recommendations following the clinic (MI-E settings if applicable or other ACTs recommended) secretion ratings using the NZSS and any medication recommendations. Descriptive statistics were used to determine the patterns observed between groups.
RESULTS
Twenty-one patients were referred to the clinic over 24 months. Ages ranged from 18–87 years (mean: 59 years). Medical diagnoses included progressive bulbar palsy (PBP) as well as multiple sclerosis, spinal muscular atrophy, and cerebral infarcts (Table 1).
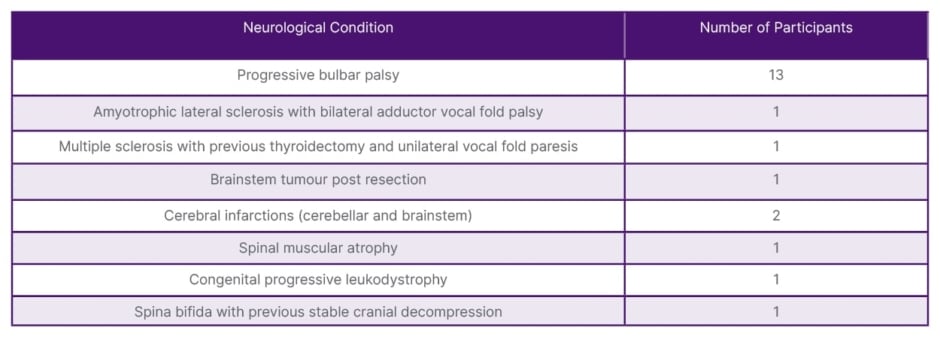
Table 1: Medical diagnosis of participants.
Severity of Bulbar Dysfunction
For patients with PBP, scores ranged from 0–8 (mean: 2.46; standard deviation: 2.90); however, the mode score was 0 (n=6), indicating the need for high levels of diet/fluid modification, which itself indicates high bulbar dysfunction. For patients with all other conditions, their average IDDSI-FDS ranged from 0–8 (mean: 2.28; standard deviation: 3.61), however; again with a mode of 0, this also indicates a need for diet modification, but to a lesser degree with some outlying patients improving this score.
ALSSS speech subscore results indicated a higher level of impairment in speech for those patients with PBP (n=13; median: 4.69) in comparison to the grouped ‘other neurological conditions’ (n=8; median: 5.5).34
Cough Augmentation Analysis
No adverse events were noted regarding laryngoscopy, and patients were considered overall to tolerate the procedure well. This procedure wielded clear guidance for individual patients and cough augmentation strategies based on visualisation at the laryngeal level. For patients with PBP (n=13), transnasal laryngoscopy resulted in discontinuation of MI-E for seven patients (54%), and changes to settings for six (46%). These changes included reduced pressures for four (67%) and insufflation for only two patients (33%) to maintain airway patency. For those patients with MI-E discontinued, unsupported breath stacking was deemed the most suitable alternative for four (31%), and supported breath stacking with the use of a lung volume recruitment bag was commenced with three (23%).
For the singular patient with limb onset ALS, vocal fold adductor palsy was identified during this procedure (the patient had no previous exposure to transnasal laryngoscopy). Given the severity of this impairment, the patient was referred for urgent discussion with the managing medical team in relation to airway management consideration. Following this finding, cough assist use was restricted to insufflation only.
In the remaining other neurological conditions group (n=7), transnasal laryngoscopy resulted in discontinuation of MI-E for one patient (14%), who was subsequently taught unsupported breath stacking; changes to settings for five patients (71%) (pressures reduced to maintain airway patency for all five); and no changes to settings for one patient (14%).
Laryngeal Response Patterns
For patients with PBP, observed hypopharyngeal/laryngeal constriction was evident across all possible structures (Table 2), the most frequent region being the arytenoids. This also interferes with vocal fold abduction and would prevent airflow into the trachea. Patients with other neurological conditions exhibited similar obstruction frequency between tongue base, hypopharynx, and arytenoids, but these were seen at higher pressures than in patients with PBP. This is suspected to be due to a higher overall bulbar impairment in patients with PBP. When hypopharyngeal (base of tongue/pharyngeal wall) or laryngeal (arytenoid or vocal fold) obstruction/closure was observed and the airway was no longer visible (Table 2), changes were made according to the algorithm (Figure 1).
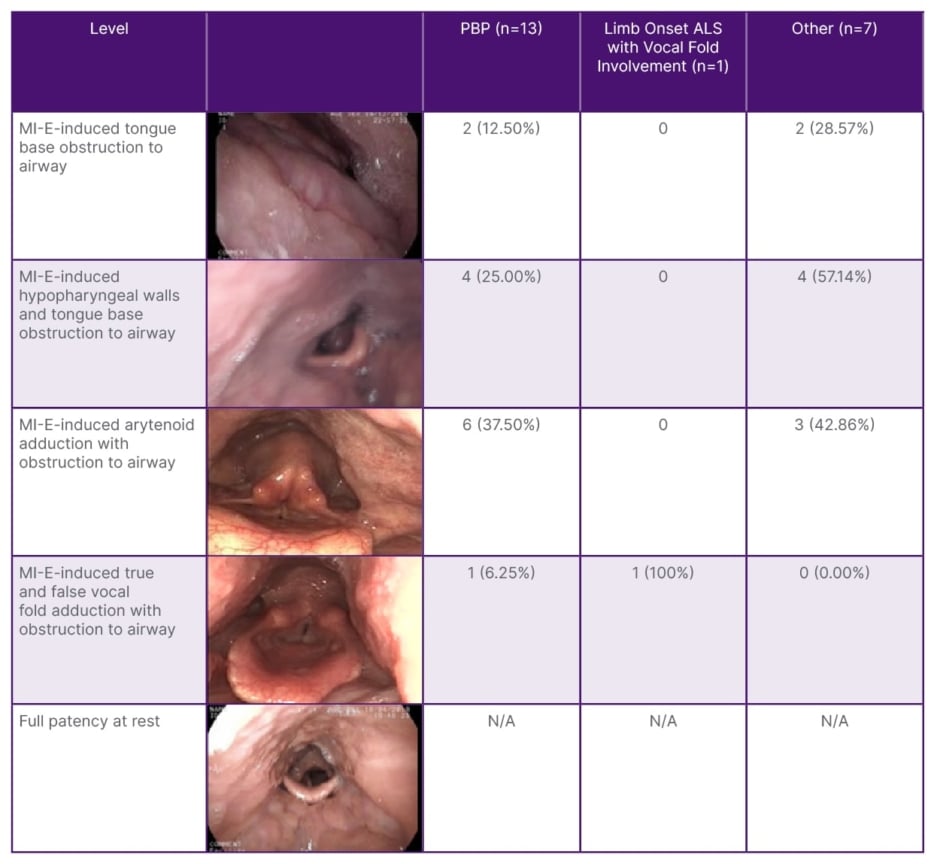
Table 2: Levels of hypopharyngeal/laryngeal changes.
ALS: amyotrophic lateral sclerosis; MI-E: mechanical insufflation-exsufflation; N/A: not applicable;
PBP: progressive bulbar palsy.
Secretion Medication Recommendations
Fifty percent of patients (n=15) scored the most severe secretion rating (7 on the NZSS), indicating accumulated secretions and dysphagia. Signs of laryngopharyngeal reflux were observed, including erythema, oedema, and posterior commissure hypertrophy. Medication changes were recommended for 47% of patients (n=7) across all conditions based on signs of laryngeal mucosa changes related to reflux (proton pump inhibitors), and lower and upper airway secretion management deficit. For secretions originating from the trachea, mucolytics or regular nebulisers were recommended, and for salivary secretions, antimuscarinics or consideration of botulinum toxin injection to the salivary glands (as appropriate) were discussed with the MDT.
DISCUSSION
This study examines the findings and outcomes of using laryngoscopy to observe the laryngeal closure patterns induced by MI-E, and any potential clinical implications in patients with varied neurological conditions and bulbar impairment.
The sample size in this study was small, and patients were pre-selected for MI-E; therefore, conclusions regarding the efficacy of MI-E are made cautiously. The team acknowledges the method of pressure increase used (2–5 cm H2O increments) could lead to patient fatigue should high pressures be reached; however, relatively low-pressure limits were observed, thus reducing the risk of procedural fatigue influencing observations. They also recognise that observations within the hypopharynx or larynx may not fully demonstrate clear patterns or trends. However, their understanding of the effects of MI-E on the larynx has been expanded and this visualisation tool has now become standard practice within the service. In addition, this work has improved the ability of the physiotherapists to understand issues patients may be experiencing based on subjective and objective bedside assessment.
Whilst visualisation resulted in changes in management for all patients, it is important to note that they could find no clear pattern in level of constriction or adduction, pressures, or choice of alternative cough augmentation strategies recommended following visualisation. Again, this is congruent with the work of other clinician researchers who reported the importance of individualised regimes for this cohort.27 One pattern that did emerge was that average pressures above +25/-25 cm H2O in the PBP group were shown to result in shutdown across all participants, which is important for clinicians to take into consideration with bedside set-up in this patient group.
Treatment efficacy was improved; however, the team are yet to confirm the functional impact of these changes on the medical status in relation to longer-term secretion management or chest infection rate. They would therefore recommend that future studies collect outcome data including chest infection rate, quality of life/patient-related scales, and compliance with MI-E in relation to hospitalisations, to assess for trends or patterns concerning bulbar impairment and functional outcomes for this cohort.
Not all services have ready access to transnasal laryngoscopy for this purpose, but the authors hope that by building on previous evidence, this study further highlights the link between bulbar impairment and MI-E tolerance, and allows clinicians to consider that higher pressures may not be beneficial for this cohort and may, in fact, result in a more negative outcome.
This study highlights the benefits of direct visualisation using transnasal laryngoscopy to optimise MI-E for patients with neurological conditions with bulbar impairment. This adds to the previous work by Anderson et al.,20 which looked at normal responses and patients with ALS and spinal onset.26-28 The work has proven safety in transnasal laryngoscopy for patients with bulbar onset and more severe bulbar impairment with an attempt to quantify the severity of their bulbar impairment. Differences in laryngeal changes between patient cohorts support the understanding of the appropriateness of MI-E for these patients. Characteristics such as hypopharyngeal and laryngeal closure help to inform and explain clinical observations. This will direct the team’s patient selection and assessment protocols moving forwards and contribute to the conclusion of previous researchers that individualised recommendations with visualisation are essential for optimisation of MI-E.40
CONCLUSION
Application of MI-E with direct visual feedback using transnasal laryngoscopy is a safe and effective method for determining the appropriateness of cough augmentation and most effective MI-E settings in patients with a range of neurological conditions with bulbar impairment. Cough augmentation strategies can be tailored accurately for patients with a variety of neurological conditions with bulbar impairment based on the results of transnasal laryngoscopy.







