Meeting Summary
At the 7th Congress of the European Academy of Neurology (EAN), held virtually in June 2021, real-world data on the efficacy and safety of opicapone (ONGENTYS®) in patients with Parkinson’s disease (PD) were presented across four posters. This evidence confirms previous findings from the Phase III clinical trial programme. The use in “early fluctuators” provides significant benefits to patients with PD in terms of efficacy and tolerability; therefore, opicapone should be considered as an early adjunctive treatment to levodopa as soon as motor fluctuations are diagnosed.
Introduction
PD is a neurodegenerative, chronic, and progressive disease. The clinical manifestations usually start after the age of 50 years, but the majority of cases are diagnosed in patients aged 60 years or above. In the USA, an estimated 60,000 people are diagnosed with this neurodegenerative disorder each year and about 1 million Americans have the condition.1 The European Parkinson’s Disease Association (EPDA) estimates that 1.2 million people have PD in the European Union (EU).2
Opicapone is a third generation, once-daily catechol-O-methyltransferase (COMT) inhibitor developed to fulfil the need for a more potent, longer-acting COMT inhibitor, with an improved safety profile.3,4 COMT inhibitors are an established first-line treatment strategy to manage motor fluctuations5 and are the only adjunct class to directly resolve the variations in plasma levodopa levels that lead to wearing-off fluctuations.6 Opicapone has improved peripheral tissue selectivity to reduce the risk of toxicity. It works by decreasing peripheral levodopa’s conversion rate into 3-O-methyldopa, thereby prolonging the duration of levodopa’s effect in reducing the ‘off’ time period of PD and extending the ‘on’ time period.7 Opicapone is approved for use as an adjuvant therapy to preparations of levodopa/ dihydroxyphenylalanine decarboxylase inhibitor in adults with PD, who are experiencing end-of-dose motor fluctuations and cannot be stabilised on those combinations, in several countries including the USA, EU, UK, Japan, South Korea, and Australia.8-11
The approval of opicapone was based on evidence from 38 clinical studies, including two multinational Phase III clinical studies (BIPARK-1 and BIPARK-2). BIPARK-1 (NCT01568073),12 a randomised, double-blind placebo- and active-controlled study, was conducted in 106 sites in 19 European countries. Approximately 600 patients with PD and end-of-dose motor fluctuations received once-daily doses of opicapone (5 mg, 25 mg, or 50 mg), placebo, or 200 mg of entacapone (a second-generation COMT inhibitor) for 14–15 weeks as adjunct to levodopa therapy. BIPARK-2 (NCT01227655),13 followed a similar study design, with approximately 400 patients taking once-daily doses of opicapone (25 mg or 50 mg) or placebo and conducted in 71 multinational sites. These studies demonstrated that opicapone consistently and significantly reduced ‘off’ time and increased ‘on’ time without increasing the frequency of troublesome dyskinesia.14 Real-world clinical practice data are needed to complement the evidence from these trials.
At EAN 2021, the post-hoc analyses from the OPTIPARK trial were presented in four posters, assessing the effectiveness of opicapone in patients with PD and motor fluctuations in the real-world setting.
Methods
These post-hoc analyses of OPTIPARK, prospective, open-label, uncontrolled, single-group, multicentre trials, were conducted in a heterogeneous population with PD treated under real-world conditions in Germany and the UK. At baseline, all patients with PD experiencing ‘wearing-off’ motor fluctuations received opicapone 50 mg once daily for a 3-month period in Germany and for a 6-month period in the UK, in addition to their current antiparkinsonian treatment. Eligible patients were male and female, aged ≥30 years with idiopathic PD, and Hoehn and Yahr I–IV at ‘on’ state and showing signs of ‘wearing-off’. The primary efficacy endpoint was the Clinician’s Global Impression of Change (CGI-C) after 3 months of treatment and secondary endpoints included: Patient’s Global Impression of Change (PGI-C), 8-item PD-Questionnaire (PDQ-8), Unified Parkinson’s Disease Rating Scale (UPDRS), and Non-Motor Symptoms Scale (NMSS). Patients were also assessed for treatment-emergent adverse events (TEAEs).15
Patients were assessed by disease duration (PD duration cut-off: <8.4 years or ≥8.4 years), onset of motor fluctuations (onset: <1 year or >1 year), baseline use of entacapone (NO or YES), and daily use of levodopa (dose: <500 mg or ≥500 mg).15
Results
Four hundred and ninety-five patients received at least one dose of opicapone, with 393 patients completing 3 months of treatment (50 mg once-daily at bedtime) for 3 (in German sites) or 6 months (in UK sites).15 Among treatment completers, baseline demographics and characteristics are shown in Table 1.16-19
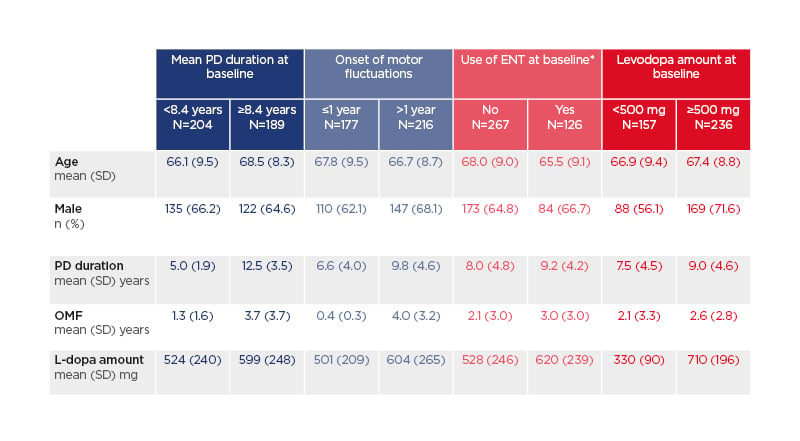
Table 1: Baseline demographics and characteristics among treatment completers (n=393).16-19*Entacapone users were required to discontinue ENT at baseline.
ENT: entacapone; L-dopa: levodopa; PD: Parkinson’s disease; SD: standard deviation.
At 3 months, patients with shorter PD duration (<8.4 years versus ≥8.4 years), more than 1 year since the onset of motor fluctuations (OMF; <1 year versus >1 year), not on entacapone at baseline (NO versus YES), and taking a lower levodopa daily dose (<500 mg versus ≥500 mg) experienced numerically greater improvements on CGI-C and PGI-C (Figure 1).16-19
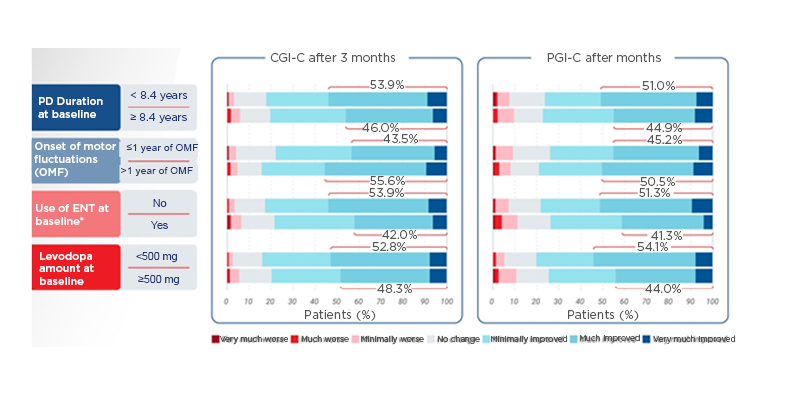
Figure 1: Clinician’s Global Impression of Change and Patient’s Global Impression of Change improvements at 3 months among treatment completers (n=393).16-19
*Entacapone users were required to discontinue ENT at baseline.
CGI-C: Clinician’s Global Impression of Change; ENT: entacapone; OMF: onset of motor fluctuations; PD: Parkinson’s disease; PGI-C: Patient’s Global Impression of Change.
The UPDRS is a rating tool used to gauge the course of PD in patients and includes series of ratings for typical PD symptoms that cover all of the hindrances of PD. The UPDRS consists of six segments: mentation, behaviour, and mood; activities of daily living; motor examination; complications of therapy; a modified Hoehn and Yahr Scale; and Schwab and England Activities of Daily Living (ADL) scale.20 These post-hoc analyses found improvement in the UPDRS scores Part II (at the ‘on’ stage) and III (motor examination), independently of PD duration (<8.4 years and ≥8.4 years) and onset of motor fluctuations (at ON stage but not on UPDRS III; <1 year versus >1 year). Those not using baseline entacapone (NO versus YES) and taking a lower levodopa daily dose (<500 mg versus ≥500 mg) experienced numerically greater improvements in both outcomes (Figure 2).16-19
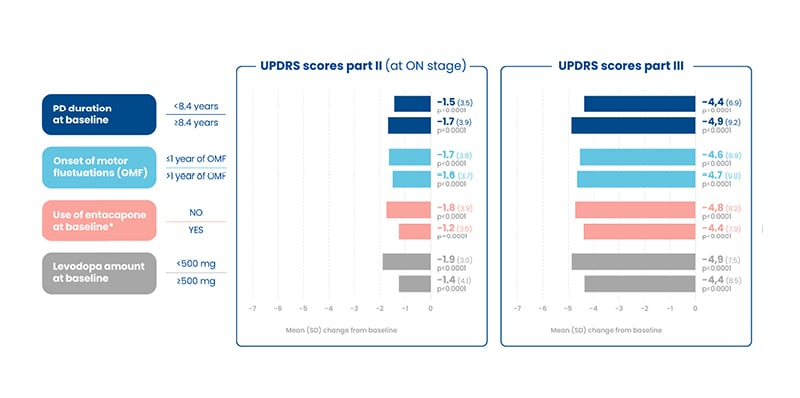
Figure 2: Unified Parkinson’s Disease Rating Scale scores, part II and III, improvements at 3 months among treatment completers (n=393).16-19
*Entacapone users were required to discontinue ENT at baseline.
ENT: entacapone; OMF: onset of motor fluctuations; PD: Parkinson’s disease; SD: standard deviation; UPDRS: Unified Parkinson’s Disease Rating Scale.
Furthermore, an analysis of quality of life (QoL), as assessed by the PDQ-8 and Non-motor Symptoms Scale (NMSS), found greater numerical improvements in patients with shorter PD duration (<8.4 years versus ≥8.4 years), less than 1 year since OMF (<1 year versus >1 year), not using baseline entacapone (NO versus YES), and taking a lower levodopa daily dose (<500 mg versus ≥500 mg). In patients experiencing recent fluctuations (≤1 year of OMF) versus those with >1 year of OMF, the mean change in PDQ-8 was -4.2 and -2.8, and in NMSS it was -7.7 and -6.1, respectively (all p<0.05 versus baseline). Furthermore, those taking a low levodopa daily dose experienced greater numerical improvements in QoL (PDQ-8) and NMSS. The mean change in PDQ-8 was -4.1, -3.0, -3.7, and -2.6; and the mean change in NMSS was -7.4, -6.4, -7.3, and -4.8 in those taking a levodopa dose of <500 mg, ≥500 mg, <750 mg, and ≥750 mg at baseline, respectively.16-19
The incidence of any and possibly related TEAEs was lower in patients with shorter PD duration (<8.4 years versus ≥8.4 years), more recent OMF (<1 year versus >1 year) and taking a lower levodopa daily dose (<500 mg versus ≥500 mg), and similar regarding baseline entacapone use (NO and YES).16-19 In the overall study, most TEAEs emerged within the first week, but were consistently low from Week 3 onwards.21
Conclusion
In conclusion, the occurrence of dyskinesias and motor fluctuations is a major problem in the long-term management of patients with PD; these can significantly impact the burden of disease on patients with increased disability and reduced QoL.22 Opicapone is a potent, long-acting, and highly selective COMT inhibitor.3,4 The data presented at the 7th EAN Congress provides real-world clinical evidence to complement the robust evidence from pivotal clinical trials. These post-hoc analyses provide support of the benefits of earlier initiation of opicapone to patients with motor fluctuations. Opicapone use in earlier stages was suggestive of equal or better effectiveness and tolerability compared with later use. Furthermore, opicapone is taken once a day, which may enable a simplified regimen when taken with levodopa compared with other COMT inhibitors. The efficacy of levodopa, when initiated early in the treatment paradigm in combination with opicapone, enables more patients living with PD to benefit from a better QoL for longer.16-19








