Abstract
Migraine is a leading cause of disability worldwide. Despite increasing knowledge about its pathophysiology and neurobiology over recent times, treatment options for both acute attacks and longer-term attack prevention were largely developed for other conditions. This has led to treatment often being complicated by side effects and compliance issues, in addition to at best only between 40 and 50% of patients having good responses to daily preventive treatment.
There is a pressing need to reduce the burden of migraine, in an era where there have been no substantial breakthroughs in treatment approved and licensed for migraine since triptans in the early 1990s. Over recent times, preclinical migraine models, clinical human migraine models, and functional neuroimaging have provided novel insights into the underlying neurochemical systems at play in migraine and have enabled more targeted research into particular molecules or receptors of particular interest.
There have been several targeted therapeutic avenues explored recently through preclinical research and clinical trials, both for abortive and preventive treatment of migraine. These have largely focussed on targeting the calcitonin gene-related peptide receptor, with small agent antagonists and monoclonal antibodies, targeting the serotonin 5-HT1F receptor by way of preventing pain without causing vascular side effects, and emerging neuromodulatory options for acute and preventive treatment. These new and emerging treatment options will be the focus of this review.
INTRODUCTION
Migraine is one of the world’s leading causes of disability.1 It is estimated that the cumulative lifetime prevalence of migraine is around 43% in women and 18% in men,2 and given the majority of those affected are young, working people, the disorder leads to significant socioeconomic burden.3 Despite the implications of migraine as a disease, the disability it causes is often under-appreciated because it does not impact life expectancy, nor is easily visible; yet, it can pose a significant clinical and public health problem.
At present the only migraine specific abortive therapies that are available to patients are the serotonin 5-HT1B/1D receptor agonists: triptans.4 These agents are used when an attack starts to attempt to stop the pain quickly; therefore, in general they are only used when pain is present. Sumatriptan was the first triptan to be developed in the late 1980s4 and was licensed in the 1990s in most countries for the acute treatment of migraine; it changed the lives of many affected.5 Further drugs within this class (including zolmitriptan, eletriptan, almotriptan, rizatriptan, and naratriptan) followed.6 However, despite significant efficacy in a proportion of patients, their use is complicated by contraindications in cardiovascular and cerebrovascular disease, as well as systemic vasoconstrictor side effects that can affect tolerability.7 With regard to preventive therapy in migraine (i.e. treatment that is taken regularly to try to prevent pain occurrence or at least reduce the frequency and/or severity of pain when it does occur), all of the medicines currently used were developed for other conditions including epilepsy, depression and hypertension. Due to the poor specificity of these agents for any migraine specific mechanism, their use is often complicated by side effects, drug interactions, compliance issues, and, at best, clinical efficacy in reducing headache frequency or severity in 50% of those with a good response.8,9
Given these challenges, there is a substantial need for targeted and effective abortive and preventive treatment in migraine to reduce the burden associated with this condition amongst those affected. Insights from preclinical10 and clinical models,11 including human provocation and functional neuroimaging,12 have helped us further our understanding about the areas of the brain, networks, neurotransmitters, and neural proteins that may be involved. These have included the identification of the role of the brainstem as an important area of the brain that is likely one of the first areas of involvement in migraine, before ascending and descending connections from cortex and other brain areas ensue,13 as well as the identification of neuropeptides and neurotransmitters that may be involved through this understanding, such as calcitonin gene-related peptide (CGRP),14-22 pituitary adenylate cyclase activating protein 38 (PACAP38), and nitric oxide.11 Such developments have fuelled targeted therapeutic research, because targeted therapies are less likely to cause adverse events. This is an exciting time for migraine research and therapeutics, and translational ‘bench to bedside’ research has been invaluable in identifying novel treatment options for further research.13
This review will focus on the main encouraging developments in the field of migraine and outline the background of how these agents were hypothesised to be effective and the clinical research leading us to where we are today. These chosen treatments were selected because of our belief in their likely impact on clinical practice and patient care in the near future, because of their stage in clinical trials, and because they have the most positive evidence of efficacy and paucity of adverse events in our opinion. It would not be possible to cover all the emerging treatments for migraine in this review, therefore for brevity and relevance to the general neurologist or general physician, this review will focus on treatments with positive controlled trials that may be seen in clinical practice in the near future.
THE CALCITONIN GENE-RELATED PEPTIDE PATHWAY
CGRP was first discovered to play a role in migraine biology in the 1980s, when preclinical and clinical studies showed elevated circulating levels during acute attacks, which returned to baseline after effective migraine treatment.14-16 CGRP’s distribution within the brain has subsequently been mapped, and it has been shown to be expressed in areas known to be important in the migraine process (such as the trigeminal ganglion and the trigeminocervical complex). Additionally, CGRP is a potent migraine trigger when administered intravenously to human migraineurs.21
Such work demonstrated the role of CGRP in migraine and has led to clinical trials, some of which are ongoing, into the use of agents targeted against CGRP and its receptor for use in both acute and preventive management of migraine.22
Gepants: Calcitonin Gene-Related Peptide Receptor Antagonists
Small molecule agents targeted against the CGRP receptor have the suffix ‘gepant’. Six molecules have been developed, but hepatotoxic concerns led to the discontinuation of development of telcagepant23-29 and of MK-3207.30 Positive results were gained from Phase IIa trials in BI4437031 and BMS-927711 (rimagepant),32 but further trials have not been presented. Phase III studies in ubrogepant (MK-1602) are ongoing after a positive Phase II dose-ranging study.33 Phase II studies with atogepant (MK-8031) are ongoing. The findings of the ongoing studies will clarify whether the hepatoxicity seen with telcagepant and MK-3207 is drug or mechanism dependent; current data all point to the former. The agents and their state of development are summarised in Table 1. Interestingly, the gepants seem to lack the vascular side effects of the triptans.27,34-37
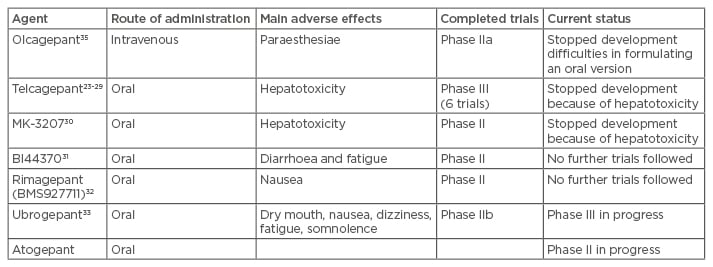
Table 1: Summary of the seven gepant drugs which were developed for migraine.
The gepants are a promising drug class, as they lack the vasoconstrictive side effects of triptans and are as effective as triptans in stopping the acute pain during migraine attacks. They would offer an attractive acute therapy for migraine that is easier to deploy as they are mostly available in oral formulations.
The Calcitonin Gene-Related Peptide Monoclonal Antibodies
Monoclonal antibodies have become an attractive therapeutic option in recent times and been developed through therapeutics research, primarily in oncology38 and rheumatology.39 More recently, they have featured in disorders of the nervous system as infrequently dosed treatments, which may help control chronic diseases, such as multiple sclerosis.40 Humanised antibodies do not have the same immunological reactions as animal antibodies when administered to a human host, are receptor specific, have long half-lives, and allow infrequent dosing compared to other agents used for prevention in chronic disease.41 Additionally, monoclonal antibodies are not renally or hepatically excreted, due to their large molecular size, and are therefore unlikely to produce hepatotoxic side effects. They are thought to be proteolytically broken down into peptide fragments and amino acids which are then used for further protein synthesis.42
Four CGRP mechanism-targeted antibodies have been developed for migraine, and all of them are currently in clinical trials for the treatment of episodic or chronic migraine. The agents and their current status are summarised in Table 2.
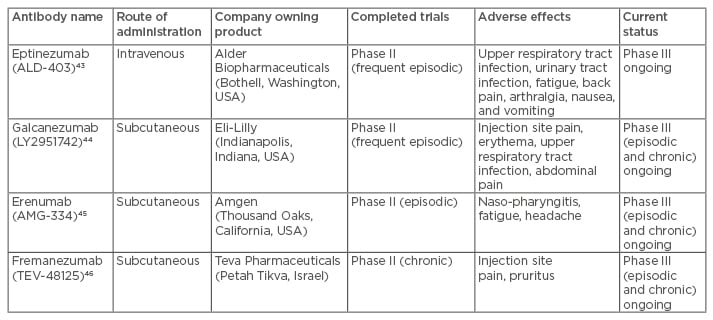
Table 2: Summary of the monoclonal antibodies targeting CGRP that have been developed for
migraine treatment.
CGRP: calcitonin gene-related peptide.
Eptinezumab (ALD-403)
This antibody is currently in a Phase III trial, following a positive Phase II study using a 1,000 mg intravenous dose.43
Galcanezumab (LY2951742)
There was a positive Phase II study using fortnightly subcutaneous dosing of 150 mg for 12 weeks, with a comparison with placebo.44 There are three ongoing Phase III studies in migraine: two in episodic and one in chronic migraine.
Erenumab (AMG-334)
A positive Phase II study in prevention of attacks in episodic migraine45 has led to two Phase III trials in episodic migraine and a Phase III and open label extension trial in chronic migraine.
Fremanezumab (TEV-48125 or LBR-101)
This antibody is currently undergoing Phase III studies in chronic and episodic migraine after a positive Phase II study in chronic migraine.46
These agents provide a promising preventive treatment option for migraine. Again, they have not shown any cardiovascular side effects,43,46-49 and side effects largely seem to be related to administration and immunological reactions, most commonly injection site reactions and upper respiratory tract infections.50 Additionally, the infrequent dosing, yet sustained effect, is attractive to patients and physicians alike, particularly for regular yet infrequent preventive use to limit the number and/or severity of migraine attacks. Further studies will provide more information about their use. Limitations in the future will include cost and identifying which patients are likely to respond. Remarkably, the CGRP pathway studied agents have all yielded positive results to date and we believe that the drugs targeting this pathway are likely to enter clinical practice in the next few years.
TARGETING THE SEROTONIN 5-HT1F RECEPTOR
The first agent developed targeting the 5-HT1F receptor specifically was LY334370. Unfortunately, it was not further developed, despite demonstrating clinical efficacy, due to liver toxicity issues and long-term safety concerns.55 The stem name for drugs targeting this receptor is ‘ditans’.The most effective anti-migraine treatments that are available for acute attacks at the moment are triptans, which were developed in the late 1980s,51 and dihydroergotamine (DHE), which was first identified in the 1940s.52 These agents are all 5-HT1B/1D receptor agonists and some also target the 5-HT1F receptor.4 By virtue of the 5-HT1B receptor agonism, they cause vascular constriction,53 both in the cranial and extra-cranial vasculature.7 It is clear from experimental studies that the 5-HT1F activation reduces trigeminovascular nociceptive activation without vascular effects.53,54 This is a promising mechanism for migraine, given that triptans are contraindicated in patients >65 years and cannot be used in a proportion of patients who have pre-existing cardiac or cerebrovascular disease, or are at high risk of it. Intravenous DHE treatment poses similar problems, and whilst it is an effective treatment option for some patients, it does not apply to everyone and carries logistical and cost implications. This leaves a large cohort of migraineurs without effective treatment when pain starts, particularly as other painkillers, such as non-steroidal anti-inflammatory agents, also bring their own issues when used in the elderly or those with vascular disease.
The first agent developed targeting the 5-HT1F receptor specifically was LY334370. Unfortunately, it was not further developed, despite demonstrating clinical efficacy, due to liver toxicity issues and long-term safety concerns.55 The stem name for drugs targeting this receptor is ‘ditans’.
Currently lasmiditan (COL-144), another specific 5-HT1F receptor agonist, is undergoing Phase III studies for the acute treatment of migraine attacks. Two Phase II studies have demonstrated clinical efficacy56,57 compared to placebo, without clear vasoconstrictor side effects. The main adverse events in these studies were dizziness, paraesthesiae, fatigue, vertigo, somnolence, and limb heaviness. The full results of a Phase III study conducted in the USA are awaited, although the initial report at the European Headache Migraine Trust International Congress (EHMTIC) 2016 was positive, and two trials are ongoing in the USA and Europe at present, one looking at response in one attack and one open label extension looking at treatment of several attacks.
This mechanism provides another promising acute abortive avenue for migraine treatment, as there is a need for effective and tolerable agents to treat the acute attack, as well as to prevent it.
NON-INVASIVE NEUROMODULATORY OPTIONS
As discussed, use of many of the migraine preventive therapies available are complicated by side effects and tolerability issues, particularly because, at the moment, all of the treatments are generally used daily. Additionally, their interactions with other drugs and influence on other disease conditions can be a challenge. With acute treatments, the vascular side effects of triptans, and the gastrointestinal and renal effects of non-steroidal anti-inflammatory agents continue to pose challenges, as does medication overuse as a potential problem, leading to inadequate analgesia for the majority of migraineurs most affected by their condition.
Such challenges have led to increasing interest in the use of neuromodulatory options for migraine management, both for acute and preventive use. In general, the neuromodulatory devices are used daily for attack prevention, and can also be used as needed for attack treatment, meaning that the patient has one single treatment strategy for their migraine. In recent times, invasive options have lost favour because of the risks of surgery and subsequent complications. For this reason, these modalities will not be discussed here. Concerns regarding the complications of invasive neuromodulation have led to the development of non-invasive devices which administer neuromodulatory therapy in a non-invasive, portable way, and treatment can be self-administered by patients themselves after brief training. In general, these options tend to be free of many of the disabling cognitive and mood side effects of the tablet preventives, and can be a useful strategy in patients in whom side effects, tolerability, polypharmacy, and drug interactions are a problem. Single pulse transcranial magnetic stimulation (sTMS) has also been shown to be safe in pregnancy,58 providing a treatment option for women who may otherwise have difficult to control headaches.
Single Pulse Transcranial Magnetic Stimulation
The rationale for using sTMS in migraine comes from evidence that abnormal corticothalamic connections exist in brains affected by migraine, and that sTMS can modulate cortical activity, as well as have an effect on cortical spreading depression, the electrophysiological correlate of migraine aura.59
sTMS in migraine has been studied in one sham controlled trial and with an open label experience using the SpringTMS device (eNeura, Maryland, USA).60,61 This device is currently only available in the USA and in the UK (via prescription), and has been approved by the National Institute for Health and Care Excellence (NICE) since January 2014.62 An open-label post-marketing study is complete and the initial data presented at EHMTC 2016 was positive. In general, it seems to have good efficacy in treating acute attacks and is well tolerated, safe to use, and can be used in pregnancy. Treatment is delivered in pulses administered over the occipital cortex using a handheld, non-invasive device. The studies using sTMS are summarised in Table 3. From our clinical experience, despite limited literature evidence,61 sTMS is also best used preventively as well as acutely, as the neuromodulatory preventive effect tends to build up over time and confer additional benefits. A clinical trial formally assessing preventive use is in progress.63
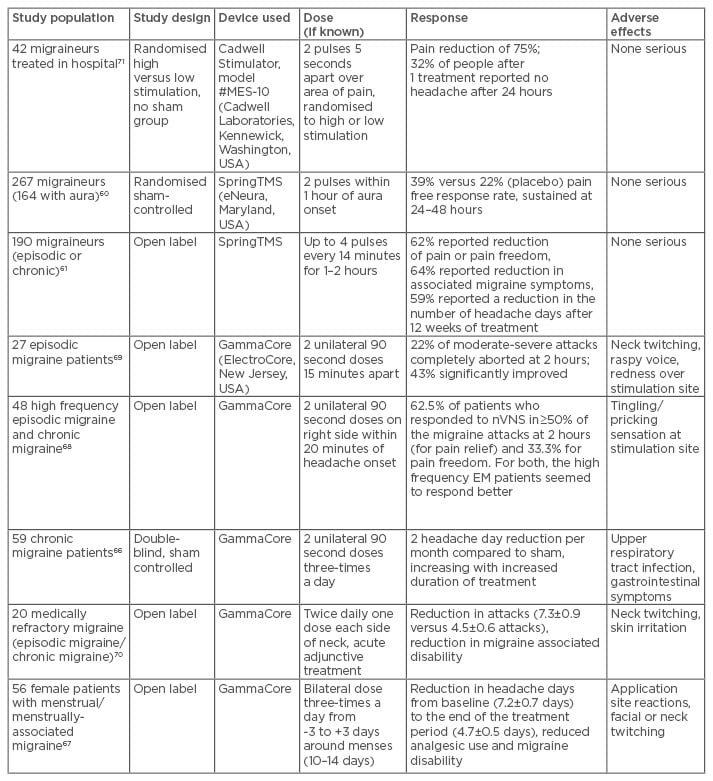
Table 3: Summary of the trials using sTMS and nVNS in migraine.
sTMS: single pulse transcranial magnetic stimulation; nVNS: non-invasive vagal nerve stimulation.
Non-Invasive Vagal Nerve Stimulation
The vagus nerve is a mixed motor and sensory nerve with autonomic function, and has anatomical and physiological connections to major pain centres of the brain, including the thalamus, suggesting a possible treatment target in migraine. Interest in this area first came from observation of a patient who was treated with vagal nerve stimulation for epilepsy and noticed a beneficial effect on his migraine.63
A handheld non-invasive vagal nerve stimulator (Gammacore, ElectroCore, New Jersey, USA) is available for purchase in many countries, including in the UK and Europe. It has also received NICE guidance supporting its use.65 Studies in migraine, both for attack treatment and prevention, have been promising, and Phase III studies are ongoing.66-70 The adverse effect profile is favourable, with only mild effects such as temporary skin erythema at the site of stimulation, transient voice hoarseness, and neck twitching being reported. The main studies using non-invasive vagal nerve stimulation are summarised in Table 3.
For both sTMS and non-invasive vagal nerve stimulation, further sham controlled, double-blind randomised studies will provide additional answers regarding efficacy, but, so far, the results in a subset of patients are encouraging.
CONCLUSIONS
There are currently more clinical trials in progress than ever before in migraine. The approaches are, for the first time, targeted against different parts of the migraine process, both for acute pain and preventive treatment. Therapeutics research in this area has come from ‘bench to bedside’ work, identifying novel therapeutic targets, and this is likely to continue in the future, with ongoing translational work from preclinical models identifying physiological targets, receptors, and ion channels of interest.
Non-invasive neuromodulatory options are already in use in the UK with promising results. These devices seem to be effective acutely but are, in general, more effective when use is sustained. Trials regarding the preventive use of these devices are ongoing. CGRP and serotonin 5-HT1F agents are likely to follow, making their way into practice, as the results of ongoing Phase III studies emerge. The CGRP antibodies may provide preventive treatment that works with monthly dosing, and a favourable side effect profile and the gepants and ditans for acute treatment devoid of vascular side effects.
This is an exciting period in migraine therapeutics and for the first time we have options to offer those most affected by this disabling condition. These and future targeted therapies are likely to be the way forward in migraine therapeutics, as there is a need for effective, well tolerated agents lacking systemic side effects for patients disabled by this common and often misunderstood condition.








