Abstract
Cannabinoids have been studied for their role in the treatment of epilepsy for many years. The U.S. Food and Drug Administration (FDA) approved them for the treatment of some refractory syndromes in 2018. Cannabidiol and tetrahydrocannabinol are the most commonly studied cannabinoids and have been studied in great depth vis-à-vis their pharmacokinetics and pharmacodynamics. Studies have shown the efficacy of cannabinoids in the treatment of refractory epilepsy. A substantial amount of research has been performed exploring the interactions between cannabinoids and other conventional antiseizure medications. The exact mechanisms by which cannabinoids exert their effects on seizure control remain unclear and research into these mechanisms continues in great earnest. Cognitive changes from cannabinoids are constantly being studied and add to potential benefits from the use of these compounds. Cultural and social misconceptions and roadblocks about the use of cannabinoids persist and represent an ongoing obstacle to increasing research and therapeutic use of these compounds. This review focuses on all these aspects and of the use of these cannabinoids in the treatment of epilepsy and seeks to offer a fairly comprehensive description of the facets of cannabinoid therapy for refractory epilepsy.
INTRODUCTION
Cannabidiol (CBD) comes from Cannabis sativa, which is a medicinal plant known to have several properties. Several types of extracts from cannabis can be broadly classified into psychotropic and non-psychotropic compounds, and CBD falls within the non-psychotropic compounds. In recent years, it has been found to be useful in several diseases such as Huntington’s disease, amyotrophic lateral sclerosis, Parkinson’s disease, and Alzheimer’s disease.1-4
Cannabis has been in medicinal use for a long time, but it was first described in the United States Pharmacopoeia in 1850.5 However, on 25th June 2018, Epidiolex® (cannabidiol) was the very first cannabis-derived drug that was approved for use.6 Sativex, which is also a derivative and is known as nabiximols, is approved as an adjunctive treatment in multiple sclerosis in several countries.7
People with epilepsy constitute 1% of the global disease burden of disease, affecting over 50 million people worldwide.8 While there are several types of epilepsy, drug treatment-resistant epilepsy is defined as having failed at least 2 antiepileptic drugs (AEDs), which were tolerated and appropriately chosen to achieve sustained seizure freedom.9 Most types of epilepsy are managed with antiepileptic drugs but for severe drug-resistant epilepsy, other treatments are being explored; one being the use of CBD.
Initially, as the medicinal use of cannabis started being explored, there were hardly any trials and evidence of efficacy was unclear. Due to the abuse potential of cannabis, many states were hesitant to approve the cannabis containing CBD. Now, with more trials and evidence, the use of CBD is being medically prescribed and distributed. Therefore, now more than ever, there is a need to increase awareness around appropriate use of CBD in various diseases, especially epilepsy.
The objectives of this review article are to describe the science behind the properties of CBD and tetrahydrocannabinol (THC), summarise the clinical trials and adverse effects to date and explore the future directions of treatment using CBD and THC.
BASIC SCIENCE
THC and CBD act via the endocannabinoid system. The endocannabinoid system is composed of G protein-coupled receptors, endogenous cannabinoid (CB) receptor ligands such as N-arachidonylethanolamine (anandamide) and 2-arachidonoylglycerol, and ligand metabolic enzymes such as fatty acid amide hydrolase and monoacylglycerol lipase.10 CB receptors are part of the GPCR family. CB1 receptors are located primarily in central and peripheral neurons, and CB2 receptors predominantly in immune cells.10 The activation of the CB1 receptors prevents excessive neuronal excitation in the central nervous system by modulation of neurotransmitter release.10 They modulate release of various inhibitory as well as excitatory molecules (transmitters) such as γ-aminobutyric acid (GABA), noradrenaline, acetylcholine, dopamine, serotonin, and glutamate.10 Endocannabinoids also act as retrograde synaptic messengers. Certain neurotransmitters can cause increase in postsynaptic calcium, which can trigger synthesis and the release of endocannabinoid molecules into synapses, which then act on presynaptic CB1 to inhibit the release of neurotransmitters such as glutamate and GABA.10 THC has equal or higher affinity towards CB1 and CB2 receptors but has lower efficacy than the other phytocannabinoids.10
While the psychotropic agent THC acts on CB1 and CB2 receptors, CBD does not. However, its actions include inhibitory action on the orphan G protein-coupled receptor 55, equilibrative nucleoside transporter, and the transient receptor potential of melastatin type 8 channel.11 On the contrary, it enhances action of the 5 hydroxytryptamine receptor 1A on the glycine receptors α3 and α1, and the transient receptor potential ankyrin type 1 channel.11 It has a bidirectional action on intracellular calcium.10
At higher concentrations, CBD exerts its excitatory effects on transient action potentials vanilloid Type 1 and Type 2 and on the nuclear peroxisome proliferator-activated receptor γ and inhibits cellular uptake and fatty acid amide hydrolase–catalysed degradation of anandamide.12
The complex combination of these receptor-ligand interactions has helped CBD emerge with antiepileptic, neuroprotective, and anti-inflammatory properties.
Pharmacokinetics
Absorption
Inhaled cannabinoids exhibit similar pharmacokinetics to intravenous (IV) cannabinoid. After inhalation, peak plasma concentrations of both THC and CBD are attained rapidly (within 3–10 min).13
Bioavailability
The bioavailability of THC has been found between 10–35%. This could be attributed to inhalational characteristics, size of inhaled particles, and inter- and intra-subject variability.13 The inhaled version of CBD has 35% bioavailability.13 The sublingual route is also noted to have much higher bioavailability than the oral form.14 Although less frequently used, the intravenous version of THC has found to have a higher bioavailability.13
Half-life
The mean half-life of CBD was reported as 1.1- and 2.4-hours, following nebuliser and aerosol administration (20 mg).15,16
Distribution
THC is highly lipophilic. Upon delivery, it gets distributed in highly perfused regions first including the lung, heart, brain, and liver.13 Mean volume of distribution was 2,520 L following IV administration.17 Apparent volume of distribution after oromucosal spray was 26,298, 31,994, and 28,312 L.18 Volume of distribution of CBD via the IV route has been found to be 32.7 (8.6) L/kg.16
Metabolism
Hydroxylation of THC at C9 by the hepatic Cytochrome P450 enzyme system leads to production of the equipotent metabolite 11 hydroxy-Δ9-THC. Cytochrome P450 2C9, 2C19, and 3A4 are involved in the oxidation of THC.19 Phase II metabolism of the 11-Nor-9-carboxy-Δ9-THC involves the addition of glucuronic acid and, less commonly, of sulfate, glutathione, amino acids, and fatty acids via the 11-carboxylic acids group.13
Although similar to THC, CBD undergoes oxidation of C9 to the alcohol and carboxylic acid and side-chain oxidation.13 Both THC and CBD are subjected to a significant first-pass effect; however, unlike THC, a large proportion of CBD is excreted, unchanged in the faeces.13
Elimination
Of the THC that gets excreted, 80–90% is excreted as hydroxylated and carboxylated metabolites within 5 days.20 More than 65% is excreted in the faeces, approximately 20% being eliminated in the urine.21
Ujváry et al. also reported that 16% of CBD administered IV was excreted in urine as unchanged and conjugated CBD in 72 hours.22 It was also observed that 33% of the initial CBD was mostly excreted as unchanged CBD, with metabolites such as mono- and di-hydroxylated and monocarboxylic derivatives of CBD in the faeces within 72 hours.22
Keeping the above properties in mind, it was found that cannabinoids have variable properties depending on the type of formulations that could be effective. To counter hurdles of first pass metabolism and poor water solubility or absorption, several synthetic compounds have been designed such as water-soluble CBD powders, self-emulsifying delivery systems, and encapsulation of CBD within gelatine matrix pellets.23
CLINICAL STUDIES
Clinical Efficacy in Current Use
To date, there have been five major Phase III randomised controlled trials (RCTs) and several open label, expanded access studies that evaluated the efficacy and safety of CBD (Tables 1 and 2).
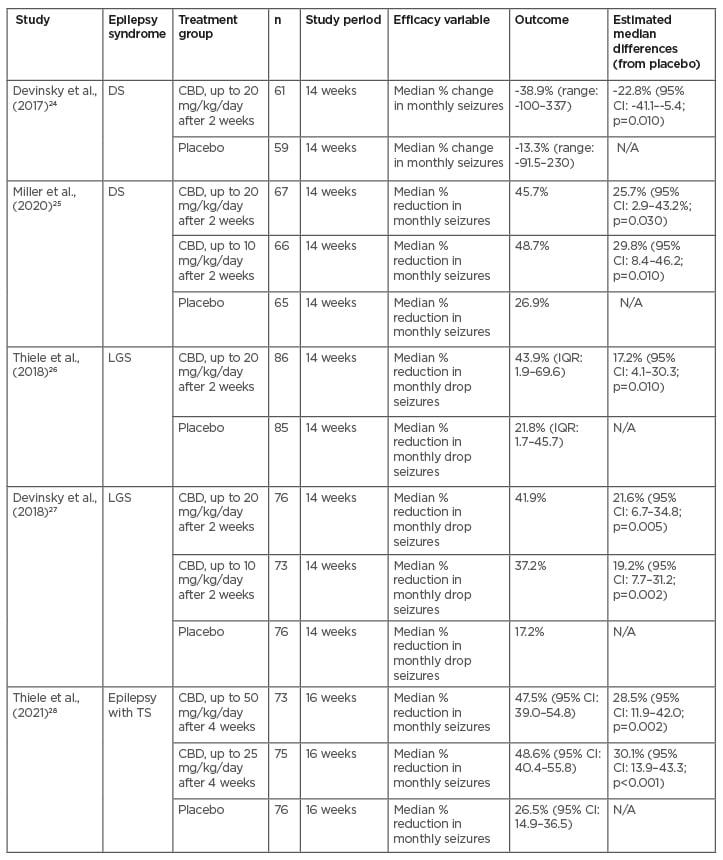
Table 1: Randomised controlled trials.
CBD: cannabidiol; CI: confidence interval; DS: Dravet syndrome; IQR: interquartile range; LGS: Lennox-Gastaut syndrome; TS: tuberous sclerosis.
Of the RCTs, two of them studied the safety and efficacy of CBD on Dravet syndrome (DS), two on Lennox-Gastaut syndrome (LGS), and one on epilepsy associated with tuberous sclerosis (TS).24-28 All RCTs were double-blind and placebo-controlled and took place in multiple centres in the USA and Europe, with the TS trial also including patients from Australia. They all demonstrated a significant difference in percentage reduction of monthly seizure frequencies over the course of at least 3 months compared to the placebo. Two of the studies assessed efficacy, comparing doses of CBD at 10 mg/kg/day and 20 mg/kg/day for DS and LGS and found that both dosing groups demonstrated significantly increased efficacy in reducing seizures compared to the placebo but otherwise produced similar results compared to each other.25,27 Adverse effects were noted to be more frequent in the higher dose group.25,27 One study similarly compared doses of CBD at 25 mg/kg/day and 50 mg/kg/day for patients with TS, both of which reduced seizure frequency compared to the placebo but otherwise produced similar results compared to each other.28
Multiple open label and expanded access trials were also identified from the literature review that demonstrated reduction in seizures in patients with various treatment-resistant epilepsies (Table 2). Six of the studies evaluated add-on treatment of CBD for treatment-resistant epilepsy (TRE) in general, one for LGS, one for epilepsy associated with TS, and one for epileptic spasms.29-37
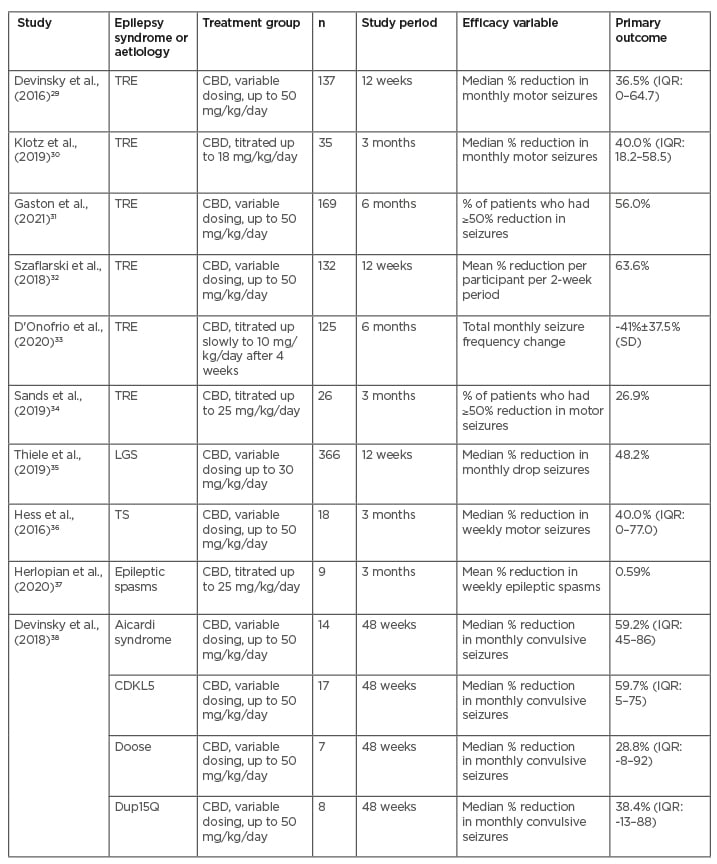
Table 2: Open-label studies.
CBD: cannabidiol;CDKL5: cyclin-dependent kinase-like 5; IQR: interquartile range; LGS: Lennox-Gastaut syndrome SD: standard deviation; TRE: treatment-resistant epilepsy; TS: tuberous sclerosis.
One study was specific to epilepsies associated with Aicardi syndrome, cyclin-dependent kinase-like 5 (CDKL5) deficiency disorder, Doose syndrome, and Dup15Q syndrome.38 These studies took place in the USA and/or Europe. In Asia, one study retrospectively evaluated the use of CBD in 42 patients with DS or LGS in South Korea and found that 33.3% of patients who received CBD at a dose of 10 mg/kg/day had at least a 50% reduction in seizure frequency after 3 months.39
Combined Effects with Clobazam
Additional studies have been performed to investigate the potential additive effects of CBD and clobazam (CLB). Using a mouse model, Anderson et al. showed that CBD and CLB can modulate the GABAA receptors to a greater extent when combined, providing a potential additive effect to their therapies, but did not act in a synergistic manner.40 In a Phase II clinical trial, VanLandingham et al. evaluated the levels of drug metabolites in blood samples of patients receiving concomitant CBD and CLB and reported that there was no evidence of any drug-drug interaction between CBD and CLB, but CBD did increase one of the metabolites of CLB.41 This study determined that CBD at a dose of 20 mg/kg/day had an acceptable safety profile while co-administered with CLB.
Gunning et al. performed a meta-analysis of the four RCTs that investigated CBD as add-on therapy for LGS or DS and calculated that patients who received the CBD add-on therapy had a significant reduction in frequency of drop seizures in LGS (treatment ratio: 0.70; 95% confidence interval (CI): 0.62–0.80; p<0.0001) and convulsive seizures in DS (treatment ratio: 0.71; 95% CI: 0.60–0.83; p<0.0001) compared to placebo.42 They then performed a subgroup analysis of patients on CLB, demonstrating a similar reduction in frequency of drop seizures in LGS (treatment ratio: 0.56; 95% CI: 0.47–0.67; p<0.0001) and convulsive seizures in DS (treatment ration: 0.63; 95% CI: 0.52–0.77; p<0.0001) compared to placebo.42
Devinsky et al., as part of the project team that carried out the RCTs, also performed a meta-analysis of the same four RCTs and found that the CBD treatment groups with (treatment ratio: 0.51; 95% CI: 0.52-0.68: p<0.0001) and without CLB (treatment ratio: 0.85; 95% CI: 0.73–0.98; p=0.0226) were more efficacious than the placebo group in average reduction in seizure frequency.43 Furthermore, they reported via logistic regression analysis that the odds ratio of patients yielding a >50% reduction in seizures from baseline was 2.51 (95% CI: 1.69–3.71; p<0.0001) in the CBD group without CLB and 2.40 (95% CI: 1.38–4.16; p=0.0020).43 The treatment ratio appeared to numerically favour the CBD group with CLB versus without CLB, but the odds ratios of achieving >50% reduction in seizures were similar.
In a separate study, Savage et al. retrospectively analysed data from 47 patients with refractory epilepsy who received CBD therapy and compared the outcomes between those who had concomitant CLB (n=32) and those who did not (n=15), finding no significant difference in reduction of mean weekly seizure frequency between the two groups.44
Investigations of CBD and Other Epilepsies
Infantile spasms
There are some clinical data available for the use of CBD in infantile spasms. In a multicentre Phase II clinical study, Hussain et al. reported that, of the 9 patients with infantile spasms refractory to vigabatrin and adrenocorticotropic hormone, after 2 weeks of receiving CBD titrated up to 20 mg/kg/day, one of the patients achieved complete response to treatment.45 This was defined as freedom from infantile spasms or hypsarrhythmia on 24-hour video EEG monitoring on Day 14 of the treatment. Similarly, in an open-label study, Herlopian et al. reported that, of the 9 patients with epileptic spasms (as classified per the 2001 International League Against Epilepsy [ILAE] at the time) who received CBD titrated up to 25 mg/kg/day, 3 of the patients were seizure-free and had resolution of the hypsarrhythmia pattern on video EEG after 2 months of treatment.37
Genetic and developmental epilepsies
Multiple preliminary clinical studies have been performed evaluating the use of CBD in other genetic and developmental treatment-resistant epilepsies as well. Devinsky et al. reported results of an open-label study using CBD for treatment-resistant epilepsy in patients with Aicardi syndrome, CDKL5 deficiency disorder, Doose syndrome, and Dup15q syndrome, reporting a 48-week median monthly convulsive seizure reduction of 59.2%, 59.7%, 28.8%, and 38.8%, respectively.38 Kuchenbuch et al. reported that 3 patients with SYNGAP1 epileptic encephalopathy had an average 85% monthly seizure reduction by Month 9 after receiving maximum doses of 10, 17, or 23 mg/kg/day.46 Poisson et al. studied the use of CBD titrated up to 30 mg/kg/day as add-on therapy in 4 patients with migrating focal seizures associated with KCNT1 mutations and found, while none of the patients following 12 weeks of treatment did not have a reduction in seizures, one of the patients had a reduced intensity of seizures.47 Kaplan et al. studied 4 paediatric patients with refractory seizures in Sturge–Weber syndrome who, at Week 14 reported an average 65% monthly reduction in seizures.48 Sands et al. reported results of an expanded access program using CBD in 26 children with various treatment-resistant epilepsies, mostly presumed to be genetic, of whom 26.9% had a ≥50% reduction in motor seizures following 3 months of treatment.34
Adverse Effects
The safety of CBD has also been extensively studied. In the five Phase III RCTs, there was a higher percentage of adverse events in the CBD groups compared to placebo groups.24-28 The most common adverse events attributed to CBD reported in these trials included somnolence, pyrexia, upper respiratory tract infection, vomiting, decreased appetite, and diarrhoea.24-28 Three of the RCTs mentioned that 3.5–12.0% of patients in the CBD treatment group who had liver transaminase levels 3 times greater than the upper limit of normal, resulting in withdrawal from the trial.24,25,27 The other two RCTs noted 13.4–18.9% of patients with elevated liver transaminase levels, most of whom were also taking valproic acid.26,28 Despite these adverse events, most patients in the CBD treatment group were able to continue receiving CBD in at least the 14–16 week treatment periods of the study. The three RCTs that compared different doses of CBD also demonstrated a higher prevalence of adverse events with higher dose groups (around 90% of patients with at least 20 mg/kg/day and 100% of patients in the 50 mg/kg/day groups) without improved clinical efficacy.25,27,28
Beyond RCTs for epilepsy, Dos Santos et al. reported a review of 18 clinical trials that included CBD as a treatment group, reiterating the common adverse effects, as mentioned above.49 They also noted that the presence of elevated transaminases, pyrexia, and upper respiratory tract infections appeared to be more frequent in patients receiving CBD as add-on therapy for seizures.49 This could be related to certain comorbidities involved or drug interactions with other antiseizure drugs. Indeed, multiple studies have demonstrated that CBD affects serum levels of multiple antiseizure drugs due to shared metabolic pathways.50-52
Given CBD’s close relationship with marijuana, some studies have also investigated its cognitive effects. In one study, Gaston et al. evaluated 20 patients with TRE who received functional MRI (fMRI) before and >2 weeks after receiving CBD with titration up to 25 mg/kg/day, while testing immediate and delayed memory, and found that treatment in CBD resulted in no significant changes in working memory performance and significant increases in neural activity on functional MRI in regions associated with verbal memory and attention compared with healthy controls.53 The same study group also explored cognitive functioning after long-term use of CBD in both children and adults with TRE and, by using the National Institute of Health (NIH) Toolbox Cognition Batter test before and after 1 year of CBD use, there was no significant change in cognitive test performance.54, 55
DIFFICULTIES IN DEVELOPMENT AND USE
Adverse Effects and Drug-drug Interactions
As discussed above, adverse effects and drug-drug interactions are a limiting factor in the initiation and continuation of CBD in some situations. Elevation in liver enzymes was the most common reason for discontinuation in the four RCTs for LGS and DS. Most, if not all, of these patients were on concurrent valproate, and there were no cases of drug induced-liver injury.42,49 Additionally, concomitant use of CBD and CLB has been associated with increased somnolence. This combination has also resulted in rare cases of pneumonia and respiratory failure, thought to be secondary to the CBD-induced increase in plasma CLB levels as opposed to CBD alone.43,49 Special attention should be paid to these interactions, especially in European populations where the use of CBD has only been approved as an adjunctive treatment with CLB. This regulatory caveat may yield to potential harm in this regard and does not allow for minimising AED burden in patients who could potentially respond to CBD independent of CLB. Studies have shown mixed results on the effects of CBD on other AEDs, specifically having either no effect on or increasing levels of valproate and topiramate.49 There have also been some reported increases in the drug levels of rufinamide, zonisamide, eslicarbazepine, and brivaracetam.49,52,56 All of this must be taken into consideration when prescribing CBD to patients with TRE who, by definition, are already on multiple other AEDs. Further studies are needed to explore potential interactions between CBD and other AEDs in order to identify potential limitations on dose escalation, minimise adverse effects, and optimise seizure control and ultimately quality of life.57
Stigmatisation
In general, stigmatisation stemming from social, political, and legal factors has been a barrier to the investigation and prescribing of medical cannabis over the years.58 While CBD is not psychoactive, given its derivation from Cannabis sativa, this stigma still carries over and raises theoretical concerns, particularly regarding abuse potential. A single dose, randomised, crossover trial demonstrated that CBD had a significantly low abuse potential at both therapeutic and supratherapeutic doses as compared to alprazolam and dronabinol in a population of recreational polydrug users.59 This study also showed that CBD had no observable cognitive or psychomotor impairment in contrast to alprazolam.59 The growing body of evidence demonstrating the efficacy and safety of CBD in the treatment of epilepsy has effectively reduced this stigma among the medical community, though some reservations may remain among patients based on cultural, political, and religious ideals. For instance, as recreational use of marijuana is forbidden in Islam, despite religious scholars considering medical use of cannabis and its derivatives acceptable, cultural barriers to patients who are Muslims accepting this as a treatment option persist.60 A survey on patient experiences with stigmatisation related to the use of medical cannabis found negative views of cannabis as a recreational drug, associated criminal sanctions, and using cannabis in the context of vulnerability (i.e., illness, disability) to be contributory to their sentiments.61 Ideally, the increasing legalisation and normalisation of medical and recreational cannabis products throughout the world will help break down some of these barriers going forward.
FUTURE DIRECTIONS
Further Understanding of Mechanism and Predicting Treatment Response
As discussed above, the complete mechanism of action of CBD in the treatment of epilepsy is not fully understood. Some studies have examined the relationship between CBD and patterns of neural synchronisation, and how these can be used to predict treatment response. Anderson et al. demonstrated that CBD treatment responders, as evidenced by >70% seizure reduction, had stronger network integration and segregation in β frequencies compared with non-responders.62 This study also showed that higher CBD dosage was associated with stronger network integration and segregation in Δ, θ, and α frequencies. Larger studies are needed to identify whether these findings suggest that CBD is causing these stronger brain network dynamics, or rather if stronger network dynamics predispose to treatment response. More clarification on this may identify which patients would benefit most from treatment with CBD. Additionally, further study of pharmacogenomics could help distinguish which patients are most likely to respond to CBD and identify optimal CBD and AED combinations on a tailored, individualised basis.57
Use in Other Types of Epilepsy
Given the limited U.S. Food and Drug Administration (FDA) indications for use of pharmaceutical-grade CBD, it is not currently available for the majority of epilepsy disorders. More evidence is needed to elucidate the efficacy of CBD in these other types of epilepsy beyond LGS, DS, and TS. There are a few ongoing clinical trials further examining CBD in these groups, in addition to one more novel study examining use in electrical status epilepticus of sleep.63,64 A recent systematic review of open-label studies and reports of experimental off-label use of purified, plant-based CBD suggested effectiveness in multiple other epilepsy syndromes including CDKL5 deficiency disorder, Aicardi syndrome, Dup15q syndrome, Doose syndrome, SYNGAP1 encephalopathy, Sturge–Weber syndrome, and epilepsy with myoclonic absences.65 There is also anecdotal evidence supporting efficacy beyond epileptic encephalopathies as some patients successfully supplement their prescribed anti-epileptic regimen with cannabis for improved seizure control. For example, a survey of patients at an Oregon tertiary care centre found a majority of these patients reported successful seizure reduction with use of both high-CBD strains and varied THC:CBD combination strains of cannabis.66 Given the risks associated with these products including psychoactive effects of THC and the method of ingestion, specifically smoking and vaping, these patients may benefit from a safer, regulated pharmaceutical-grade CBD option for the maximisation of their epilepsy treatment.
Antiepileptic Potential of Other Cannabinoids
The therapeutic potential of other cannabinoids in epilepsy requires further evaluation. Cannabidivarin has anticonvulsant properties in animal models, specifically in acute seizure and status epilepticus and was recently evaluated in a Phase II clinical trial for focal seizures; however, it did not meet primary endpoint of percentage change in focal seizure frequency.56,67,68 Cannabigerol did not demonstrate anticonvulsant properties in a mouse model, despite voltage-gated sodium channel blockade.69 Tetrahydrocannabivarin has been shown to suppress seizure activity in rats.70 Cannabichromene and its related phytocannabinoids were recently demonstrated to have anticonvulsant properties in a DS mouse model.71 Other cannabinoids including cannabinol, cannabidiolic acid, and tetrahydrocannabinolic acid that have been researched for neuroprotective and therapeutic potential in other neurologic conditions have yet to be studied in epilepsy.72
There is also evidence to suggest benefit from combinations of cannabinoids. A recent observational meta-analysis showed that CBD-rich cannabis extracts were over 4 times more potent as compared to purified CBD, such that the same therapeutic effect could be achieved with significantly lower doses.73 Mild and severe adverse effects were significantly lower with CBD-rich extracts as compared with purified CBD as well. These observations support hypotheses of a synergistic or ‘entourage effect’ of CBD, with other minor phytocannabinoids and suggest that plant-based CBD extracts could potentially be more efficacious and better tolerated than the currently approved purified CBD in the treatment of seizures.
CONCLUSIONS
CBD exhibits antiepileptic effects through complex actions at multiple receptors in the brain. Previously there was a deficiency of evidence to support its use in the treatment of epilepsy due to legal barriers. Now, there have been 5 RCTs and several other open-label trials demonstrating the efficacy of CBD in the treatment of LGS, DS, TS, and TRE. While these studies have yielded promising results, there were some doubts about whether this data suggested a synergistic effect of CBD and CLB, or truly represented CBD efficacy independently. Several additional meta-analyses of the major RCTs have shown similar efficacy of CBD both concomitantly and independent of CLB, though this combination does cause increased adverse effects, particularly sedation. Going forward, as use of CBD in the treatment of TRE increases, there is much more to be discovered regarding the complete mechanism of action, how to predict treatment responders, use in other forms of epilepsy, and possibly increased therapeutic potential when combined with other cannabinoids.







