BACKGROUND AND AIMS
Subthalamic deep brain stimulation (STN-DBS) is an effective treatment for motor fluctuations in Parkinson’s disease (PD). Several parameters can be controlled to improve the clinical outcomes. Among them, frequency has variable clinical effects,1 perhaps owing to the differential impact on downstream networks. High-frequency stimulation (HFS) (i.e., above 100 Hz) is classically the best choice to control the segmental PD symptoms2 and may also improve akinesia and tremor. Higher frequencies (i.e., 130–180 Hz) can further improve the tremor;1 however, they may also worsen axial symptoms in late-stage PD patients as exemplified in gait.3 Thus, there is a potential benefit to fine-tune stimulation frequency independently in both hemispheres to account for asymmetric symptom expression. Beyond directional leads4 the Cartesia-Boston® system for STN-DBS (Vercise Cartesia™, Boston Scientific, Santa Clarita, California, USA) allows configuring different frequencies of stimulation between left and right hemispheres (called differentiated frequency).5 The authors sought to explore if differentiated frequency can improve the asymmetrical tremor in patients with STN-DBS.
METHODS
Seventeen PD patients with STN-DBS (implanted with the Cartesia-Boston® system) were assessed under four conditions (stimulation on/medication off, stimulation off/medication off, stimulation off/medication on, stimulation on/medication on) 1 year after implantation. All patients participated in the PREDI-STIM study,6 a multicentre study of the predictive factors of the therapeutic response to subthalamic stimulation on the long-term quality of life in 700 people with PD. Four of these patients (Table 1) were not satisfied with their DBS outcomes because of persistent asymmetrical tremor. All patients were initially programmed with a stimulation frequency of 130 Hz bilaterally. The persistent tremor was not resolved with medication changes, increased stimulation amplitude, or stimulation on different contacts (including directional stimulation). Pulse width was reduced in two patients.
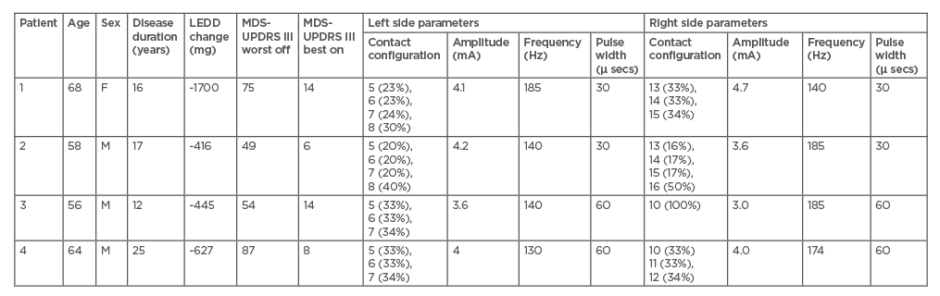
Table 1: Clinical features of the four patients and DBS parameters proposed to relieve their tremor.
Best on condition: stimulation on/medication on; F: female; LEDD change: levodopa equivalent daily dose change between pre- and post-DBS implementation; M: male; MDS-UPDRS: Movement Disorder Society-unified Parkinson’s disease rating scale; worst off condition: stimulation off/medication off.
Increasing the frequency of stimulation reduced the asymmetrical tremor, but all four patients reported worsening of the akinesia and hypertonia on the opposite side. Therefore, differentiated frequency was proposed with the higher frequency programmed contralateral to the side with the persistent tremor. To assess the efficacy of tremor reduction on the most affected side, the authors calculated a tremor subscore corresponding to the sum of the following items of the Movement Disorder Society-unified Parkinson’s disease rating scale (MDS-UPDRS) Part III: 3.15 (postural tremor of the hands), 3.16 (kinetic tremor4.0of, the hands), 3.17 (rest tremor amplitude for upper and lower limb), and 3.18 (constancy of rest tremor).
RESULTS
With differentiated HFS, (185 Hz versus 140 Hz for three patients, 174 Hz versus 130 Hz for the last one) tremor subscore decreased for three patients (Table 1). Patient clinical global impression improved one point for three patients in comparison with symmetrical HFS (130 Hz on both sides). One patient did not report any change and no worsening of the total MDS-UPDRS III
was observed.
CONCLUSION
Expanded programming flexibility with the opportunity to programme different frequencies for each side could be optimal for certain patients to reduce tremor and maintain a good control of the other symptoms. Despite this proof of concept, further clinical trials remain necessary because of interpatient variability.








