BACKGROUND AND AIMS
Amyotrophic lateral sclerosis (ALS) is an incurable neurodegenerative disease. Underlying genetic pathomechanisms include the C9orf72 hexanucleotide GGGGCC repeat expansion, the most frequent genetic cause of ALS (C9-ALS) in Western countries.¹
Neurodegeneration in C9-ALS is supposedly associated with two mechanisms: a loss- and a gain-of-function mechanism.¹ They are not mutually exclusive and depend on the site of transcription start. The former refers to reduced transcription and translation of the C9orf72 protein. The latter involves the increased transcription and translation of hexanucleotide repeat expansion, leading to the generation of foci of transcribed RNA and the accumulation of translated toxic dipeptide repeat proteins.¹ Another pathological hallmark is represented by TDP-43, a ubiquitous intranuclear protein that can translocate and accumulate in a phosphorylated form into the cytoplasm of glial cells and neurons.² Despite recent progress in unravelling C9-ALS pathogenesis, reliable disease models and disease-modifying therapies are still lacking.
Organoids refer to 3D cultures derived from induced pluripotent stem cells that present a complex cytoarchitecture with a layered structure, dynamically resembling some phases of early human development.³ Neural organoids consist of diverse cellular subpopulations, including proliferating, differentiating, migrating, and self-organising pools of neural progenitors, enabling the study of cell-to-cell communication, the patterning of peripheral and central nervous system regions, and the evaluation of neural connectivity.⁴ Their generation, coupled with genome editing, microfluidics, live imaging, and single-cell genomics, has allowed the modelling in vitro of different neurological disorders,5 including neurodegenerative diseases such as Huntington’s disease,⁶ Alzheimer’s disease,7 and Parkinson’s disease.⁸ To date, an organoid model of C9-ALS is still lacking.
Here, the authors aim to model C9-ALS in vitro using 3D human spinal cord organoids (SCOs).
MATERIALS AND METHODS
The authors differentiated C9-ALS induced pluripotent stem cells and isogenic controls using a free-floating 3D culture method with a multi-stage protocol involving various differentiation factors. They generated SCOs with a modified Lancaster’s protocol,⁹ promoting neural caudalisation (Wnt signalling and retinoic acid) and ventralisation (sonic hedgehog signalling). Long-term growth was achieved using spinning flasks. The authors treated C9-ALS SCOs with morpholino antisense oligonucleotides against C9orf72 repeat expansion. Finally, they assessed the differentiation of organoids at different time points with immunohistochemical and real-time quantitative PCR (qPCR) analysis.
RESULTS
The authors obtained isogenic and C9-ALS SCOs displaying different co-existing neuronal sub-populations at different time points (at Days 30, 45, and 80). SCOs expressed neural progenitor, pan-neuronal, astrocyte, motor neuron, and rostrocaudal markers, including markers of cervicobrachial spinal cells (Figure 1). Compared to controls, C9-ALS organoids exhibited increased dipeptide repeat proteins levels, DNA damage markers associated with C9orf72 expansion, and cytoplasmic inclusions of translocated TDP-43. Gene expression analysis using qPCR confirmed the expression of neural precursors, post-mitotic neural, and motor neuron-related genes in both C9-ALS and isogenic spinal cord organoids. Preliminary results on qPCR reported differential expression of genes associated with DNA damage and motor neurons in morpholino antisense oligonucleotides treated C9-ALS organoids.
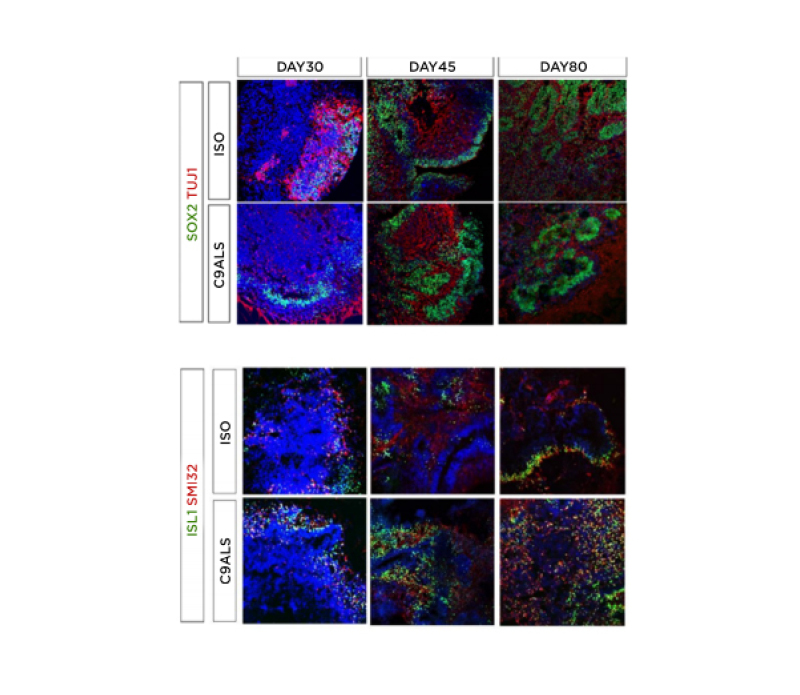
Figure 1: Immunohistochemical characterisation of C9-ALS spinal cord organoids.
Isogenic and C9-ALS spinal cord organoids stained for neuronal precursors, post-mitotic, and motor neuron markers. Time-course of SOX2/TuJ1 (neural precursor/post-mitotic) as well as motor neuron markers ISL1/SMI32 on isogenic and ALS organoids at Days 30, 45, and 80. C9-ALS: C9orf72 hexanucleotide GGGGCC amyotrophic lateral sclerosis; ISO: isoquercetin.
CONCLUSION
SCOs represent a valuable system for modelling some neuropathological hallmarks of C9-ALS, investigating C9-ALS pathomechanisms, and testing possible new treatments in vitro.








