Vagus nerve stimulation (VNS) is a neurostimulation therapy in which the vagus nerve is stimulated in the neck region by a helical electrode that is wound around the cervical vagus fibres and connected with a lead to a subclavicularly implanted pulse generator. It represents a safe and efficacious neurostimulation treatment that is now widely available for refractory epilepsy patients. With the initial VNS devices, stimulation was delivered in an open-loop fashion with intermittent stimulation of the vagus nerve in on and off cycles. On top of this standard stimulation, a magnet feature enables delivery of an extra stimulation at the time of a perceived seizure onset by passing a magnet across the implanted generator.1,2 Studies have proven the added benefit of this magnetic stimulation,2-6 but manual application of the magnet may not be possible due to clinical seizure symptoms, a physical or cognitive impairment, unawareness of the seizure occurrence, or nocturnal seizures.1,2 Therefore, the development of an automated seizure detection feature that triggers stimulation has gained interest. Since electroencephalogram and electrocardiogram studies have shown that ictal heart rate increases occur in approximately 82% of epilepsy patients,7 detection of these ictal heart rate changes could serve as a trigger to automated stimulation delivery. The combined open-closed loop VNS system is the first VNS device with a cardiac-based seizure detection algorithm, providing automatic stimulation triggered by ictal heart rate increases. This novel feature functions in addition to the traditional VNS system modes (normal and magnet mode), resulting in a potential therapy combination for patients using both open and closed-loop stimulation.1,2
In our study, we evaluated the performance and safety of this new device in a cohort of 16 refractory epilepsy patients with a minimum follow-up of 6 months. We retrospectively assessed the change in mean monthly seizure frequency (MMSF), responder rate, and adverse events at maximum follow-up. Five patients had a maximum follow-up of 15 months with a change in MMSF from 11 to 5 and a 60% response rate at 15 months. Two patients had a maximum follow-up of 12 months with a change in MMSF from 17 to 15 and a 50% response rate at 12 months. Seven patients completed a maximum follow-up of 9 months and showed a reduction in MMSF from 17 to 6 and a response rate of 43%. Finally, two patients had a maximum follow-up of 6 months with a change in MMSF from 38 to 5 and a 50% response rate. The total response rate at maximum follow-up was 50%. Less severe seizures at maximum follow-up were reported by 44% of patients. Regarding safety, the most frequently reported adverse event was hoarseness, while other adverse events were rarely reported (Figure 1). In half of the patients, the stimulation parameters had to be adjusted due to adverse events.
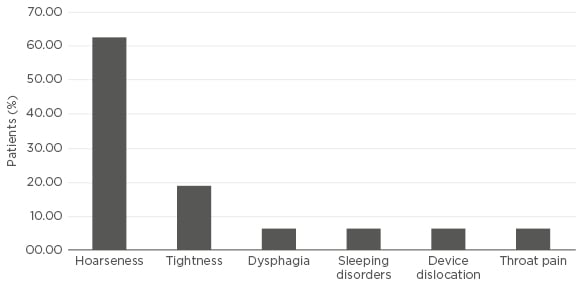
Figure 1: Adverse events at maximum follow-up.
We can conclude that the response rate with the combined open-closed loop VNS system in this study was comparable with previous short-term VNS studies. In accordance with a previous study, we found in this small group of patients a trend towards higher response rates earlier in the course of follow-up. The safety profile was comparable to the traditional open-loop VNS devices.








