Speakers: Peter Rutherford, Dieter Götte
Affiliations: Vifor Pharma, Zurich, Switzerland
Disclosure: These studies were supported by Vifor Fresenius Medical Care Renal Pharma; the authors are employees of Vifor Pharma.
Acknowledgements: Writing assistance was provided by Eleanor Roberts, Beeline Science Communications, Ltd, London, UK. The authors would like to thank all those involved in this research including Philip Spearpoint and and Nina Plötz from Vifor Pharma; Melinda Stamm, James O’Donoghue, and Xierong Liu from Elma Research, London, UK; Angelika Deichmann and Matthias Schönermark from SKC Beratungsgesellschaft GmbH, Hannover, Germany; and Marthe Teusch from Linkfluence, London, UK.
Citation: EMJ Nephrol. 2020;8[Suppl 4]:2-16.
Meeting Summary
Anti-neutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis (AAV) can affect many vital organs including the kidneys, lungs, and nervous system. A series of posters was presented at conferences in Europe and the USA in 2018 and 2019 detailing the patient journey from initial symptoms to diagnosis, treatment, and, in some, relapse. These posters included a retrospective audit of healthcare records from over 1,000 European AAV patients, a study utilising data from German healthcare records, an examination of social media use, and outcomes of patient interviews that were used to gain an understanding of the experience of an individual with AAV.
Symptoms in AAV can vary with the disease severity, from mild and localised to severe and life-threatening. Diagnosis can involve the referral of a patient between several specialities, often because of the different symptoms and comorbidities associated with AAV. Involved clinicians most often include nephrologists and rheumatologists, but may include a respiratory physician, dermatologist, cardiologist, ophthalmologist, or neurologist. Possibly because of these varying elements, patients found the lack of continuity of care to be a troubling factor in their journey with AAV.
Induction treatment is most often carried out during hospitalisation. In more severe cases, admission to an intensive care unit and mechanical ventilation may be required. Initial treatment is followed by several years of maintenance therapy, which will be altered if a patient relapses. Therapy choice includes a combination of immunosuppressant drugs and glucocorticoids (GC), and should be tailored to an individual patient’s needs. Though therapy regimens can bring symptom relief and vasculitis control, remission is variable and relapse is common, as are adverse events (AE) because of medications, especially GC. Patients expressed a desire to be more involved in treatment choice and more informed about their disease and the potential course of AAV.
INTRODUCTION
AAV encompasses a rare group of severe, progressive autoimmune diseases that involve a necrotising vasculitis and can affect many vital organs including the kidneys, lungs, and nervous system. Clinically, AAV is subdivided into granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA), diseases with a similar phenotype with main loci in the kidneys and lungs, and eosinophilic GPA (EGPA), which has a distinctive clinical phenotype and is associated with blood eosinophilia and asthma.1
AAV (GPA and MPA) has an unpredictable and variable disease course and can be life-threatening.2 Clinical symptoms range from a localised skin rash to fulminant multisystem disease. Missed or delayed diagnosis strongly influences prognosis if critical organs are involved.3 AAV is often a relapsing disease and early prediction or recognition of recurrence is particularly important because relapse can further increase organ damage and morbidity.4
A series of posters was presented at several conferences in Europe and the USA between 2018 and 2019 regarding the patient journey with AAV, including symptom presentation, diagnosis, treatment, AE profiles, potential relapse, and a peer-discussion of these topics.5–14 Six of these presentations utilised information from a retrospective clinical audit of healthcare records (RCAHR) (November 2014–February 2017) from incident (n=929) and relapsing (n=268) patients with AAV, managed by 399 healthcare professionals (HCP) in France, Germany, Italy, and the UK. HCP included 240 nephrologists (60 in each country), 120 rheumatologists (40 each in the UK, Italy, and Germany, and 20 in France), and 20 internal medicine physicians in France. Patients aged ≥18 years needed to have had GPA or MPA for ≥12 months, to have received at least one course of remission induction therapy for incident or relapsing disease with at least 6 months follow-up, and have had continuous care by the reporting HCP over the time of follow-up. The HCP completed up to three programmed patient record forms via an online data collection tool. Data were collected relating to baseline presentation with AAV or time of relapse, followed by clinical outcomes at 1, 3, 6, and 12 months. Descriptive statistics were used to analyse the data. Data were from HCP-selected patients so there was the potential of bias; in particular, there were no deaths in the cohort. Data were not assessed for quality of reporting.5–10
Overall, slightly more patients in the RCAHR dataset were male (54% of incident patients, 60% of the relapsing group) and slightly more had GPA (54%) than MPA (46%). Mean age was 56.8 (standard deviation [SD]: 14.2) years for the incident patients and 58.3 (SD: 13.1) years for those who relapsed. Most incident AAV patients were aged 46–55 (21%), 56–65 (29%), or 66–75 (20%) years. A further 9% were aged >75 years, 14% were aged 36–45 years, and 9% were 18–35 years old. Age range distribution was similar for relapsing patients, with 74% being 46–75 years old. At the time of diagnosis, most of the patients worked full (37%) or part time (15%), or were retired (32%).5–10
Longitudinal data of people with GPA or MPA for at least 3 years (between 2011 and 2016) were also collected from statutory health insurance companies in Germany, using the Institute for Applied Health Research (InGef) database. This represents around 7 million insured people, though for this study data were taken from an age- and sex-stratified representation cohort of approximately 4 million people aged ≥18 years old. From these data, prevalence of AAV in Germany was estimated to be, on average, 260 per million population with an incidence of 50 per million population.11 Prevalence increased with age, peaking in those aged 70–79 years.12
The experience of living with AAV will vary between patients and over time as the disease remits and relapses. Treatment choices and the impact, both positive and negative, of specific medications may be relevant. It is important to understand unmet patient needs and appreciate the patient perspective on clinical trials, new developments, and guideline recommendations. To gain insight into the experience of a patient with AAV, 33 patients aged ≥18 years who had been diagnosed with GPA (n=20), MPA (n=12), or EGPA (n=1) for at least 1 year participated in a 1:1 semi-structured interview. The patients were from the UK (n=10), Italy (n=9), Germany (n=8), and France (n=6), with most living in an urban setting (n=21). The majority were aged 40–60 (n=16) or 60–79 (n=13) years, with three aged <40 and one from the ≥80 years age group. Time since AAV diagnosis varied greatly (1.0–32.0 years) between participants, with a median of 3.5 years.13 Results from this study will be discussed throughout this article.
Social media is an important platform to examine because it is where patients may discuss their concerns spontaneously. Analysis was made of the scope and content of social media conversations that mentioned AAV in the UK (1,952 posts), Germany (320 posts), France (179 posts), Spain (178 posts), and Portugal (93 posts) to better understand challenges, unmet needs, and the patient experience. This translated to a high reach (estimated users exposed to the post) of 1.57 million people in the UK and a maximum of 255,000 in the other countries. Data were collected between 1st October 2016 and 30th September 2018 using the Radarly platform and a defined list of AAV-related search terms that were translated into representative languages.14
In the above analysis, websites were a common and increasingly frequent way to post in most countries, ranging from 22% of social media posts in Portugal to 43% in France. Forum use was common in the UK and Germany (30% in each) and, to some extent, France (19%). Social media sites, most notably Facebook, Instagram, and Twitter, were commonly posted to in Portugal (41%, 21%, and 1% of posts, respectively), the UK (2%, 31%, and 5%, respectively), and Spain (28%, 0%, and 6%, respectively). Many posts were also found on media pages and, to a lesser extent, blogs in Portugal (30% and 8%, respectively), France (21% and 6%, respectively), and Germany (30% and 8%, respectively). A subsample of stakeholder comments found that while around 50% of the comments arose from patients in the UK (n=105), Germany (n=69), and France (n=32), this represented <10% in Portugal (n=22) and Spain (n=29). Other contributors (not patients or HCP) contributed 34%, 57%, and 55% of comments in France, Portugal, and Spain, respectively. Far lower percentages were found in France (6%), Germany (4%), and the UK (1%).14 Findings from this study are integrated below.
DIAGNOSIS OF AAV
Patient interviews revealed that the AAV journey starts with feeling very unwell, bringing feelings of fear, confusion, frustration, and exhaustion. “[I] felt like my head would explode! [It was] unbearable,” said one patient. The path to diagnosis was sometimes long and winding and took up to a year in some cases. A key theme here was ‘suboptimal referral’. Major concerns were raised about this period, with some patients experiencing symptoms for months before diagnosis and AAV only being confirmed after seeing several different specialists. Patients reported feeling “invisible or tortured” during this period, with some, especially those without close caregivers, expressing strong emotional problems and feeling there was a lack of counselling or psychological support. These feelings sometimes led to mistrust, anger, and isolation.13
Reflecting this initial patient journey, examination of the RCAHR data showed that 75% of newly diagnosed incident AAV patients were referred to the treating physician by other HCP, as opposed to seeing the treating physician initially. While diagnosis was predominantly made by a nephrologist (30%), an internal medicine HCP (29%), or a rheumatologist (26%), referrals most often came from a general practitioner (49%) or an internal medicine HCP (21%), with some coming from a nephrologist (7%), a rheumatologist (6%), or an unnamed referral (17%).5 A subset of the RCAHR data, encompassing 717 patients, showed that those with severe disease are more often seen by a nephrologist (39.6%), with significantly fewer seen by a rheumatologist (21.5%),8 reflecting the frequency of renal involvement in AAV.1 In contrast, those with moderate disease were more often seen by a rheumatologist (62.1%) compared to a nephrologist (52.5%). A lower percentage presented with mild disease and more saw a rheumatologist (16.5%) than a nephrologist (8.2%).8
Although a few patients were diagnosed during an emergency department visit (5%) and some had their diagnosis as an outpatient (35%), the majority received their AAV diagnosis when hospitalised (61%).5 In the German InGef dataset, around a quarter of GPA patients and a fifth of patients with MPA were diagnosed in an outpatient setting. For GPA patients, this was most often by a general practitioner (around 38%), suggesting a familiarity with GPA, with MPA patients being most often diagnosed as an outpatient by a general practitioner (around 13%), nephrologist (around 13%), or rheumatologist (<10%).12
AAV SYMPTOMS AND COMORBIDITIES
Symptoms of AAV can vary greatly from one patient to the next. Figure 1 shows that nearly two-thirds of patients in the RCAHR cohort had renal disease at diagnosis, and around one-half presented symptoms as diverse as fatigue, fever, weight loss, and joint pain. Other symptoms included muscular or nerve-related pain, rash, and nasal symptoms.5
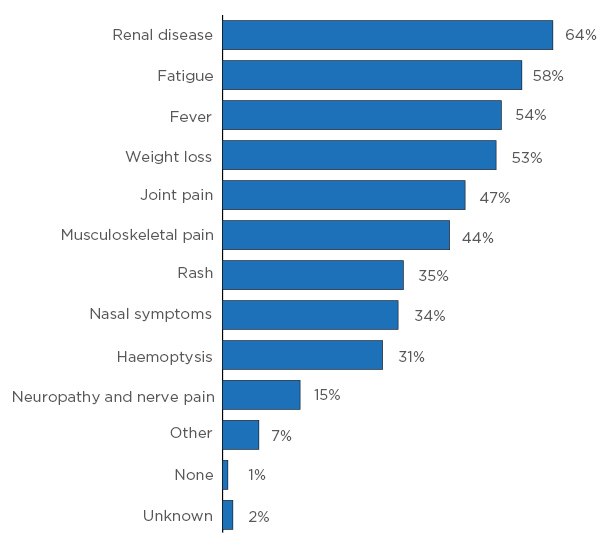
Figure 1: Symptoms at diagnosis.
Reflective of this are findings of multiorgan involvement (Figure 2A), especially in the kidneys, lungs, skin, and sinuses. Comorbidities were also often present (Figure 2B), most frequently hypertension, but also Type 2 diabetes mellitus, chronic obstructive pulmonary disease (COPD)/asthma, coronary arterial disease, and arthritis (Figure 2).5,7
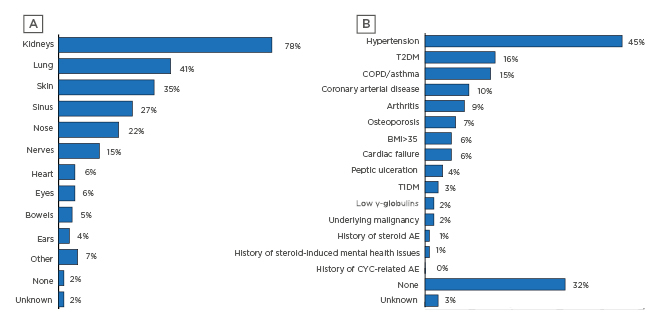
Figure 2: A) Organs involved at diagnosis and B) comorbidities at diagnosis for incident patients with anti-neutrophil cytoplasmic autoantibody-associated vasculitis.
AE: adverse events; COPD: chronic obstructive pulmonary disease; CYC: cyclophosphamide; T1DM: Type 1 diabetes mellitus; T2DM: Type 2 diabetes mellitus.
In the German cohort from the InGef database, the top 20 comorbidities were compared to a general patient population and found to be more frequent in those with AAV. Around one-half of the GPA and MPA comorbidities overlapped, including hypertension; heart failure; disorders of fluid, electrolyte, and acid–base balance; and disorders of the urinary system . For patients with GPA, other comorbidities included glomerular disorders, pneumonia, lipoprotein metabolism disorders, and other lipidaemias. For MPA patients, there was a dramatic increase in renal disorder nephritic syndrome, glomerular disorders, other disorders of arteries and arterioles, respiratory failure, and other disorders of the endocrine, respiratory, and renal systems.12
Discussion of symptoms was of particular interest for patients and carers posting on social media. Posts included discussion of their own symptoms, including ear, nose, and throat (ENT) and upper respiratory tract (URT) infection, joint pain, and fatigue/tiredness, as well as overall functional ability and the impact of symptoms on their social and daily life. For HCP, discussion centred more around long-term organ damage, ENT/URT infection, joint pain, fatigue/tiredness, and weight loss. Both groups also discussed other symptoms such as night sweats, appetite loss, and drug toxicity.14
TESTING TO DIAGNOSE AAV
Eventually, the symptoms a patient is experiencing are recognised as AAV. At this point, some patients described how they felt they were “a lucky survivor or saved,” which led to a strong bond with, and gratitude toward, the diagnosing physician. With diagnosis came relief, but also worry and anxiety. “It is a double-edged sword,” reported one patient. Such problems necessitate psychological and emotional support, counselling, and practical advice. However, the emotional burden was reported to be under-recognised and not adequately supported by HCP.13
Overall for those with incident AAV, the time between symptom presentation and diagnosis reported by physicians in the RCAHR data had a mean of 9 weeks (median: 6 weeks). The majority were diagnosed 1–4 weeks (38%) or 5–8 weeks (24%) from initial symptom presentation. However for some, diagnosis took 9–12 (11%), 13–16 (6%), or ≥17 (10%) weeks to occur.5 This is important for any AAV patient as time to diagnosis can impact long-term outcomes; for instance, it has been shown that risk of 3-year end-stage renal disease/mortality is increased in those where diagnosis takes >22 weeks.15
Diagnostic tests can include both those for AAV itself and for disease activity. In the RCAHR dataset, serological testing revealed anti-proteinase 3 (the cytoplasmic ANCA-delineating antigen) in 48.3% of patients with incident AAV, suggesting GPA, and anti-myeloperoxidase (the perinuclear ANCA-delineating antigen) in 46.6% of incident patients, predominantly found in those with MPA.3,5,9 Patient interviews revealed a number of knowledge gaps regarding diagnosis, including what caused their AAV and what their diagnosis (GPA or MPA) meant.13 This was reflected in social media analysis, where patient/carer and HCP concerns included time to diagnosis and lack of disease information.14
Diagnosis in the RCAHR dataset was often accompanied with tests of kidney function and disease (estimated glomerular filtration rate [eGFR] and proteinuria), inflammation (C-reactive protein), and assessment of AAV disease activity with the Birmingham Vasculitis Activity Score (BVAS), though this was not commonly used (Figure 3).5,9
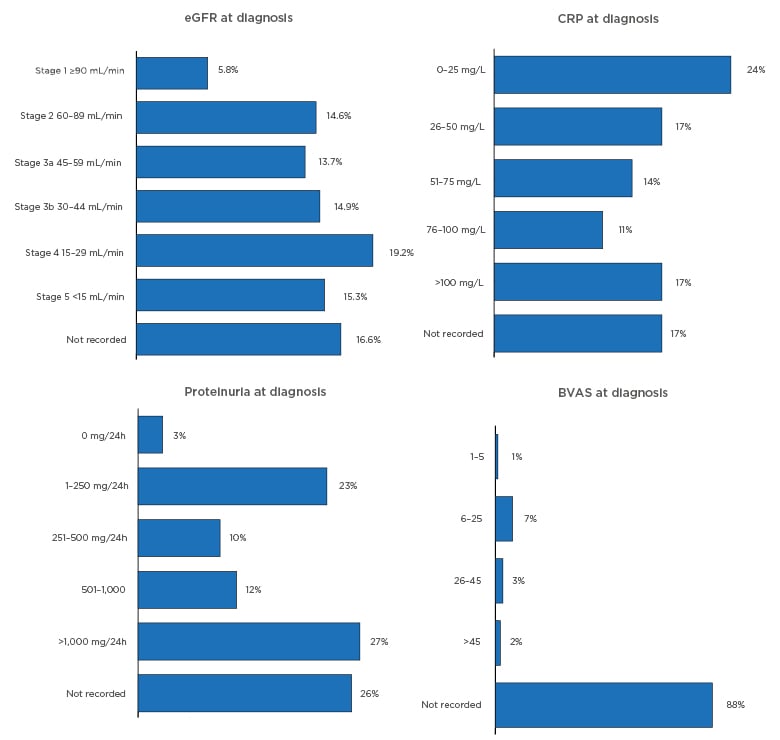
Figure 3: Diagnostic tests and investigations.
BVAS: Birmingham Vasculitis Activity Score; CRP: C-reactive protein; eGFR: estimated glomerular filtration rate; h: hour.
Urine abnormalities were common in the RCAHR patients: ≥62% had haematuria and there was a median protein excretion of 595 mg/24 hours, with 27% of patients having levels over 1,000 mg/24 hours (Figure 3).5 Histological support for an AAV diagnosis is recommended by the European League Against Rheumatism (EULAR)/European Renal Association–European Dialysis and Transplant Association (ERA–EDTA) guidelines.2 This was performed in 86% of patients in the RCAHR cohort, which found that renal biopsy was the most common procedure (64%), followed by skin (12%), and nose or sinus (11%) biopsy.5
At the start of remission induction therapy, one-third of patients with incident AAV (33.6%) in the RCAHR cohort were characterised by the physicians as having severe, rapidly progressive systemic disease. Just over one-half (54.5%) of the cohort had moderate, systemic disease, and mild, localised disease was found in 12.2% of patients.5
TREATMENT
Therapy for AAV can be complex, necessitating an individual clinical course for each patient. It is important to achieve control of AAV as soon as possible to preserve organ function; to avoid treatment-related morbidity, such as infection, while preventing longer term GC-related damage; and to decrease mortality risk.3 Interviews revealed that patients wanted a “clear treatment plan” but a common theme was lack of knowledge of the impact and duration of treatment.13
Initial remission induction treatment was most often carried out in the inpatient setting (69%), though a higher percentage in the RCAHR dataset from France were treated in hospital (76%) compared to Italy (68%), the UK (62%), and Germany (61%).5,6 The average duration of hospital stay was 15 days (median: 13 days), with most patients staying 1–7 (31%) or 8–14 (33%) days. Longer-than-average stays of 15–21 days (20%), 22–28 days (10%), and ≥29 days (7%) were reported for some patients. On average, 3 days of hospitalisation were in intensive care.5 Most patients in the German InGef dataset (around 93%) were hospitalised during the AAV remission induction therapy period, often for AAV plus a comorbid illness (45% of those with MPA, 34% of those with GPA). Patients in this cohort were frequently treated in an intensive care unit, especially those with infections and renal involvement.12 For patients who were interviewed, hospitalisation and treatment brought symptom relief, but also caused distress and isolation. While the goal was to “get out of danger,” patients experienced fear of death and were concerned about the impact of being hospitalised.13
Treatment of AAV usually involves GC and immunosuppressive drugs, often with the risk of significant AE.4 Treatment choice depends on many factors, including age, disease severity, ANCA specificity, renal function, and patient need.2–4 For patients, decision making during initial, maintenance, and long-term therapy (>5 years) was another key theme that arose; they did not feel they were as involved in treatment benefit versus risk assessments as they should be.13
High-dose GC (1 mg/kg/day up to a maximum daily dose of 80 mg) is recommended by EULAR/ERA–EDTA guidelines as part of induction therapy for AAV. This treatment scheme should be gradually tapered, to gain a low dose of 7.5 mg–10 mg/day by Week 12, though, in reality, it has been shown that the average time to achieve this low dose is 19–21 weeks.2 Initial therapy choice in the RCAHR dataset involved GC in 82.6% of those with incident AAV, cyclophosphamide for 59.2%, and rituximab for 24.4%. Other medications utilised included azathioprine (6.5%), mycophenolate mofetil (3.1%), and methotrexate (6.4%).9 Induction therapy was most often given as a combination treatment, with GC being co-prescribed with cyclophosphamide (43%), rituximab (13%), cyclophosphamide plus rituximab (3%), azathioprine (3%), or methotrexate (3%). Few patients received monotherapy with GC (10%), cyclophosphamide (7%), rituximab (5%), azathioprine (1%), or methotrexate (1%).10
Medication regimen for induction therapy was somewhat predicted by AAV severity in the RCAHR data. Of those with severe AAV, 86.8% were prescribed GC, 72.4% cyclophosphamide, and 28.4% rituximab. Corresponding rates for those with moderate AAV were 80.4%, 49.4%, and 29.6%, respectively. For those with mild AAV, respective rates were 69.6%, 23.2%, and 26.1%, respectively, with 13.8% receiving methotrexate and 8.0% receiving mycophenolate mofetil or azathioprine.8 While these data showed lower use of rituximab compared to cyclophosphamide for patients with AAV in real-world practice, there is randomised trial evidence of noninferiority for remission induction. In that trial, 197 patients with severe AAV received either rituximab (once per week for 4 weeks) or cyclophosphamide for 3.0–6.0 months (to remission), followed by azathioprine for 12.0–15.0 months plus prednisolone (tapered to end after 5.5 months if the patient achieved remission). The primary endpoint of complete remission at 6 months was reached by 64% of those in the rituximab group and 53% in the cyclophosphamide–azathioprine group. At 12 months, 48% of patients in the rituximab group and 39% in the cyclophosphamide–azathioprine group achieved sustained remission, while 39% and 33% of patients achieved remission at 18 months, respectively. This showed that, overall, the rituximab regimen was noninferior to the cyclophosphamide regimen (p<0.001). While AE did occur, leading to treatment discontinuation in 14% of those in the rituximab group and 17% in the cyclophosphamide–azathioprine group, the AE profile was similar between the groups with regard to total number of events, serious AE, and number of participants with ≥1 non-disease-related AE.16,17
In line with EULAR/ERA–EDTA guidelines,2 for the majority of patients with incident AAV, RCAHR data showed that percentages of those receiving GC dropped over the first year, from 83% at treatment initiation and 82% at Month 1, to 79% at Month 3 and 67% at Month 6. However, 53% were still receiving GC at Month 12.7,10 EULAR/ERA–EDTA guidelines suggest that GC therapy should be tapered from an initially high dose,2 and indeed, it was found that GC dosing was decreased in 31% of patients at Month 1, 45% at Month 3, 38% at Month 6, and 23% at Month 12. By Month 12, the majority of patients (56%) were taking 5–10 mg/day of GC, with only one-third (34%) taking <5 mg/day. In addition, 9% were taking GC at a dose of 11–20 mg/day and 2% were taking >20 mg/day.10 Examination of data from the German InGef database showed that 55% of patients with incident AAV were prescribed GC in the outpatient setting in the first 3 months following diagnosis, with only 6% receiving ≤7.5 mg/day. By 1 year post-diagnosis, around 40% of patients were still receiving GC.12
A concern in many countries is treatment cost. For those in the German InGef dataset, the average total cost during the 9 months following diagnosis of AAV was €28,137 and €26,137 for GPA and MPA, respectively. Subsequent cumulative costs for induction and at 3 years post-treatment were €70,641 and €94,889, respectively.11
Analysis of social media posts found that, of those discussing treatments, the majority of posts from patients/carers were with regard to GC or rituximab, with fewer regarding cyclophosphamide, azathioprine, or other drugs. For HCP, the main topics of interest were rituximab and rituximab biosimilars, with a few discussions on GC, cyclophosphamide, and other drugs.14
Response to Therapy
Though remission induction therapy is essential for patient survival, there are still significant unmet needs in AAV regarding achievement and sustainment of remission. Patients reported that the accompanying emotions at initial and maintenance treatment stages of therapy included a loss of self-image and a stage dominated by “pills and hospital visits,” bringing a long-lasting emotional impact. There was a reported feeling amongst patients of a low awareness of measures used to assess response to therapy. They expressed the desire to be told, for instance, when their “levels in blood were going down,” along with ways to express that they were “feeling better,” information on when they were “going home,” and when they would “return to normal.”13
Risk of morbidity and mortality is a central part of AAV. A recent analysis of four European Vasculitis Society (EUVAS) inception clinical trials (N=354) found that the best predictor of long-term survival is disease remission at 3 months that is sustained until at least 6 months. This was found to be the best predictor above all other disease- and comorbidity-related factors, including renal function, and was postulated to be “due to a combination of accrual of disease-related damage and drug toxicity.”18 Examination of the patients with incident AAV in the RCAHR data found a variable response to induction therapy (Table 1). A full response (no AAV activity and GC tapering on track) was seen in only 17.7% of all patients at 1 month, though this increased to 43.4% at Month 3 and 58.8% at 12 months of therapy. Most patients experienced a partial response at Month 1 (55.8%), meaning a reduction in AAV activity and an arrest of major organ damage. At all timepoints between 1 month (7.5%) and 12 months (4.8%), a minority of patients had no improvement in AAV activity (no response).7,8
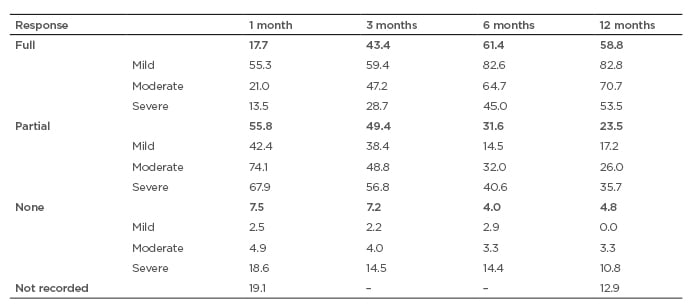
Table 1: Response to induction therapy over time according to disease severity (% of anti-neutrophil cytoplasmic autoantibody-associated vasculitis incident patients at each time point following start of induction therapy).
Response varied according to disease severity at diagnosis, with the majority of patients with mild or moderate AAV experiencing a full response after 6 or 12 months’ treatment, compared to only one-half of those with severe AAV.6-8 It was also found that the rate of hospitalisations was 11% at Month 1, with a slow decline to 10% at Month 3, 8% at Month 6, and 6% at Month 12.7
In the RCAHR data, the greatest difference in response rates was between 1 and 3 months of therapy. Analysis was also carried out to examine how response at 1 month was reflected after 12 months of treatment. Figure 4 shows that while most of those with a full initial response retained this after a year, only 58% of those with an initial partial response showed a full response at the year’s end. More hopefully, 20% of those with no response after 1 month’s therapy had a full response after 1 year.7
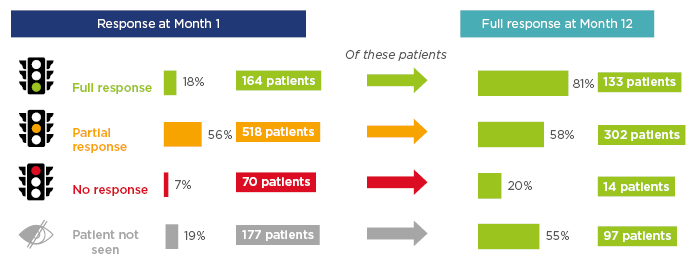
Figure 4: Response to therapy by patients with incident anti-neutrophil cytoplasmic autoantibody-associated vasculitis at 1 and 12 months.
In addition, data were collected from the patients in the RCAHR study for the most recent follow-up appointment with the physician (mean: 17.7 months since diagnosis for incident AAV patients). At the longest follow-up, vasculitis activity was found in 67.0%, moderate-to-severe systemic activity was shown in 5.7%, mild-to-moderate systemic activity in 10.5%, and local activity in 16.8%. Comorbidities at follow-up were still prevalent, of those with incident AAV, renal disease was found in 42.9%, hypertension in 42.6%, Type 2 diabetes mellitus in 16.8%, and cardiac disease in 6.6%.9
This shows that there is still a large unmet need of sustained disease control and prevention of cumulative organ damage for those with AAV.
Social media analysis found a number of unmet needs, and challenges were discussed regarding the course of AAV. For patients/carers, the greatest concerns included the impact of AAV on everyday life, mental health, and work. For HCP, concerns centred around AE and impact on work. Other subjects that arose were maintenance of remission and relapse, having a support network, and financial concerns.14
Adverse Events
AE following treatment induction, including infections and organ damage, are associated both with AAV and treatment of such.3,4,19 Particular attention needs to be paid to AE because in the German InGef dataset, patients with severe infections and pulmonary involvement showed a 1.4% and 0.5% chance of mortality per quarter (3 months), respectively.12 Patient interviews found that those treated with an immunosuppressant were concerned about understanding the risk of side effects, especially those who had been treated with such medications previously. Those patients treated with GC reported an initial energy boost, but this typically lasted only a few weeks; they also reported a number of AE including weight gain, body image changes, mental health changes (negative mood and sleep disturbance), and impaired cognition.13
The RCAHR dataset was examined to determine rates in incident patients of AE, including: i) all events excluding infections; and ii) infections. These figures were found to be 45% and 27%, respectively, at Month 1, with an average of 2.0 AE and 1.5 infections per patient, respectively. At Month 3, rates of AE and infections were 42% and 28%, respectively, with averages of 1.9 and 1.4 AE per patient, respectively. By Month 6, rates were decreased for both (35% and 23%, respectively), with slightly lower respective averages of 1.8 and 1.3. By Month 12, respective rates for AE and infections were 30% and 20%, with averages of 1.7 and 1.3, respectively.7 In the German InGef dataset, during the first 3 months of therapy, just under one-third of patients experienced a serious infection while they were being treated in hospital or that required hospitalisation. In the 1–3 years following induction therapy, incidence of severe infections was approximately 10%.12
Examination of specific AE (Table 2) shows that at all time points in the RCAHR dataset, hypertension and anaemia were most common in patients with incident AAV, followed by leukopenia and kidney disease. Infections were mostly noted in the respiratory tract or urine and remained a clinical problem for the first 12 months of treatment.10
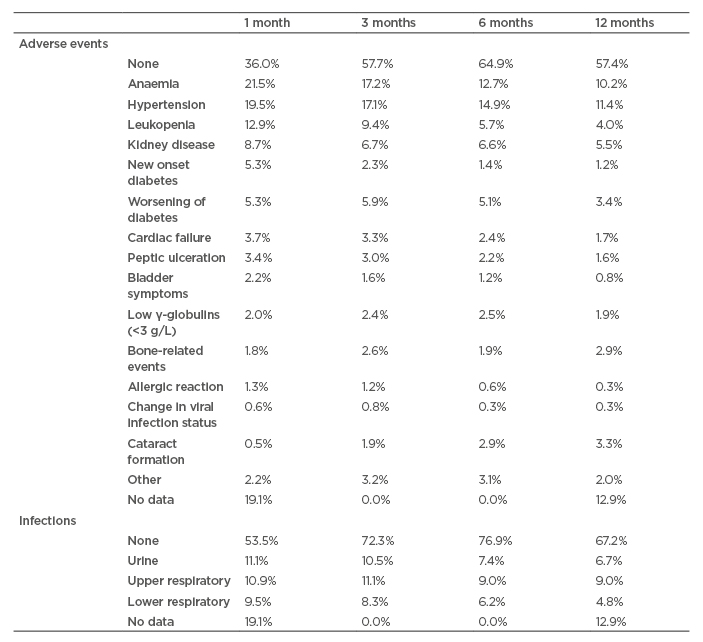
Table 2: Percentage of patients with an adverse event or infection at each time point.
Continuity of Care
Once discharged from their initial hospital stay, a patient with AAV needs to return to clinics for regular check-ups, which may necessitate the involvement of a number of different HCP depending on AAV type, organ(s) affected, and comorbid conditions. Patient interviews found that this part of their journey was sometimes frustrating because of poor communication between hospital departments.13 This is reflected in findings in the RCAHR data set showing that follow-up care was managed by one HCP in only 17% of patients, most often the physician completing the study (78%). Other involved HCP (not including the main managing physician) were a nephrologist (51%), rheumatologist (27%), internal medicine specialist (18%), or respiratory physician (16%). Other specialists that were involved (≤11%) included those associated with ENT conditions or those involved in intensive care, cardiology, dermatology, or neurology.5
Analysis of patient interviews showed a number of factors that could lead to a strong, long-lasting adverse experience and impact of AAV. These included rapid progression of AAV, accompanied by acute hospital presentation; delayed diagnosis, which could include numerous referrals and some erroneous diagnoses; and instances where care was complex, with the patient having to visit multiple HCP throughout their disease journey. A moderate, negative, but temporary, impact was reported more often when disease progression was slow, by those who received a rapid diagnosis with a quick referral to a specialist, and where there was a single team controlling AAV management.13
RELAPSE IN AAV
Discontinuing immunosuppressant medication brought feelings of “relief from the burden” of treatment for patients with incident AAV. However, patients also expressed anxiety regarding the possibility of relapsing. If it did occur, it was reported by patients to bring frustration and devastation, though they also expressed feelings of resilience and stoicism.13
Relapse in AAV remains a major clinical challenge with ≥10% patients experiencing relapses of varying severity each year following diagnosis.6 In the German dataset, over 3 years, 12% of those with GPA and 11% of those with MPA relapsed.12 Relapse may occur several times: follow-up data from the relapsing cohort in RCAHR (total duration 47.4 months; SD: 35.6) revealed that while for 62.7% of those with relapsing AAV this was their first time, for 23.1% it was the second, and for 14.2% it was the third relapse they had experienced.6
Although relapsing AAV was a reoccurrence of what had been experienced previously, in the RCAHR data diagnosis took >12 weeks in 19.8% of patients and between 9 and 12 weeks in 13.1%.6 One-quarter of those with relapsing AAV were categorised as severe AAV (25.7%), with 64.6% categorised as moderate and 9.7% as mild. Anti-proteinase 3 was found in 54.1% of patients and anti-myeloperoxidase in 46.3%.8,9
Relapsing patients are at high risk of cumulative organ damage from both acute vasculitis and drug-related AE. Similar to patients with incident disease, for those with relapsing AAV there was multiorgan involvement which most often included the kidneys (72.8%), lungs (44.8%), skin (36.2%), and sinuses (23.1%), with similar percentages reported in Figure 1 for other areas including the nerves, heart, eyes, bowels, and ears.6,14 Additionally, while eGFR was slightly higher at Month 1 for relapsing patients as opposed to incident patients (47 mL/minute compared to 45 mL/minute), it was marginally lower by Month 12 (54 mL/minute versus 55 mL/minute).14
Comorbidities in relapsing patients were also similar to those with incident AAV, though, for most, were seen in a higher percentage of patients. For instance, hypertension was observed in 52.6% of patients at the time of AAV relapse (45.0% with incident AAV), Type 2 diabetes mellitus in 23.1% (16.0% with incident AAV), COPD/asthma in 19.4% (15.0% with incident AAV), and coronary arterial disease in 16.0% (10.0% with incident AAV). Other common comorbidities, reported by 7.0–13.0% of patients with relapsing AAV, included arthritis, osteoporosis, BMI >35, cardiac failure, and peptic ulceration.6
Induction therapy for relapsing patients in the RCAHR data was found to be mainly carried out during inpatient care (69% of patients). Slightly fewer patients who relapsed, compared to those with incident AAV, received GC (76% versus 83%) or cyclophosphamide (35% versus 59%) as part of induction therapy.6,7,10 Conversely, more relapsing patients (44.0%) were prescribed rituximab than incident patients (24.4%).6,7 This therapy choice is borne out by studies showing that in those with severe relapsing AAV, more patients administered rituximab (67% of 51 patients), compared to cyclophosphamide (42% of 50 patients) (both with GC for the initial 5.5 months of therapy), achieved complete remission at 6.0 months following therapy initiation, with remission rates of 48% and 39% at 12 months, respectively.16,17
By the end of the follow-up period (total AAV duration: 47.4 months; SD: 35.6 months), GC dose in the 42.4% of relapsing patients receiving prednisone was <5 mg in 27.4%, with most receiving 5–10 mg (60.2%) and some receiving higher doses of 11–20 mg (10.6%) or >20 mg (1.8%). At follow-up, 13.4% of patients received chronic renal replacement therapy.6
Similar to results in those with incident AAV, response to remission induction therapy was variable for relapsing patients. Slightly lower proportions of patients showed a full response at Months 1 (13.8%) and 12 (54.1%) compared to incident AAV patients (shown in Table 1). While partial response was initially similar (55.8%), slightly lower percentages of relapsing patients also showed a partial response after 12 months (29.1%). ‘No response’ rates were similar at Month 1 (7.8%) and Month 12 (4.5%). Also similar to the incident AAV data, of those who showed a full response, 81% maintained this at 12 months. Rates of full response at Month 12 for those who had a partial response or no response at Month 1 were 49.0% and 38.0%, respectively. Rates of AE, including toxicity reaction but not including infections, and infections were slightly higher in relapsing patients than the incident AAV population: respective rates of AE and infection were 41% and 28% at Month 1; 52% and 30% at Month 3; 43% and 27% at Month 6; and 35% and 23% at Month 12.6
In addition, data were collected from the patients who relapsed in the RCAHR study at their most recent follow-up appointment with the physician. Follow-up data (mean: 34.3 months from start of remission induction therapy for relapse) showed that even with continued therapy, 42.7% of those with relapsing AAV still had current vasculitis activity, classed as localised in 24.3%, mild-to-moderate systemic in 24.3%, and moderate-to-severe systemic in 8.7% of patients.9 This indicates a clear, unmet need for long-term disease control and prevention of cumulative organ damage.
RENAL DISEASE
Renal disease in AAV is common (Figure 1), with many patients experiencing kidney involvement at diagnosis (Figure 2). As such, 63.9% of patients with incident AAV undergo renal biopsy as part of their differential diagnosis.9 Renal disease is denoted by a hallmark lesion described as a “destructive pauci-immune necrotising and crescentic glomerulonephritis.”20 Kidney involvement in AAV is a challenging combination of active vasculitis, cumulative damage, and an increase in risk factors for chronic kidney disease (CKD). Even with therapy, one-quarter of those with AAV develop end-stage renal disease over the 3–4 years following diagnosis.20
RCAHR data (Figure 3) showed ≥34.5% of those with AAV had Stage 4 or 5 CKD and 43.2% had Stage 2 or 3, based on tests of eGFR at diagnosis. These figures were slightly lower for those with relapsing AAV (25.0% and 53.0%, respectively), reflected in a median eGFR of 40.3 mL/minute and mean urinary protein level of 348.3 mg/24 hours compared to the incident AAV median of 34.7 mL/minute for eGFR and mean 1,489.0 mg/24 hours for protein levels. Of note, these results still indicate CKD at a median of Stage 3B for both incident and relapsing patients.5,9 Over the course of the year, average eGFR increased from 45 mL/minute at Month 1 to 51 mL/minute at Month 3 and 55 mL/minute at Month 12, reflecting an improvement in CKD staging.7
In the German dataset, severe kidney disease occurred in 19% of those with MPA and 11% of those with GPA during induction therapy. After 1 year, severe kidney disease was present in 8% with MPA and 3% with GPA, respectively. At 3 years, severe kidney disease was present in 2.5% of patients with either MPA or GPA. Most importantly, data from this study revealed that in each quarter, for 3.6% of AAV patients, severe kidney disease was fatal.12
Renal disease may necessitate longer time as an inpatient for those with AAV compared to patients with AAV without renal disease. Examination of patients with AAV in Germany showed that renal involvement required an average hospital stay of 13 days per quarter, compared to 10 days in those with an infection, 7 days in those with pulmonary involvement, and 6 days in those with GPA or MPA alone. Severe kidney disease is also a key driver increasing the cumulative cost of care, with the average cost of €131,521–€145,472 for GPA and MPA in the German dataset.11
Renal replacement therapy, which can include both dialysis and subsequent kidney transplantation, may be required for people with AAV.2 In the RCAHR cohort, renal replacement therapy was needed by 16% of patients with AAV in the first month of treatment, with some also requiring it at 3 (3%), 6 (1%), and 12 (1%) months following treatment initiation.9 Over the 3 years following diagnosis, the German dataset found renal replacement therapy varied slightly according to pathology. While 18% of those with MPA required such therapy, only 10% of those with GPA received this. Within 4 years post-diagnosis, 2.5% of patients with MPA and 0.8% of those with GPA received a kidney transplant.12
As the occurrence of renal disease in those with AAV can be the result of active vasculitis, early and sustained remission could be important in renal protection.18 In fact, as previously discussed, renal outcomes are worse in patients whose diagnosis is delayed.15 More severe vasculitis is associated with more severe renal damage; for instance, microscopic haematuria was seen in 77% of patients with severe vasculitis, both incident and relapsing, at the time of commencing remission induction therapy, versus 29% of those with mild vasculitis. The same patients with severe vasculitis had a lower eGFR (median eGFR: 25.9 versus 71.4 mL/minute) and a higher median urinary protein level (1,480.0 versus 348.3 mg/24 hours) compared to patients with mild vasculitis at this time.9
As noted, with remission induction therapy, eGFR increased for both incident and relapsing patients over the course of a year in the RCAHR dataset.6 However, follow-up data showed ongoing renal vasculitis activity at the most recent clinic visit in just over one-half of those with relapsing AAV (50.9% at 34.3 months follow-up) and 42.9% of those with incident AAV (17.7 months follow-up).9 This indicates that for those with renal involvement, continuous monitoring and treatment is necessary to manage ongoing glomerular inflammation and vascular damage causing progressive renal damage.
CONCLUSION
Patients with AAV often have a complex pathway to the HCP, who makes the correct diagnosis because of multiorgan disease with complex symptomatology and comorbidities. As such, patients with AAV experience many challenges before and after diagnosis and the emotional impact can be long-lasting. Vasculitis is systemic and often severe, and therapy is delivered in a cross-specialty manner in many cases.
Response to remission induction therapy is variable and, while early response is associated with better rates of complete response at 12 months, a high proportion of patients have only a slow and partial response. Therapy-related AE and infections are common, especially in the first 3 months, and most patients will experience at least one of these problems in the first 12 months of remission induction treatment. As such, there are unmet medical needs in patients with AAV related to improving full, rapid, and sustained response rates and reduced toxicity of therapy. Relapse is still common in patients with AAV and those who relapse face many unmet medical needs, including time to relapse recognition and remission rates.
There is an evolution of patient experience over time but challenges of recognition of their views, information gaps, and involvement in decision making are recurrent and important themes. The fear of relapse and the repeated need for induction therapy is a particular concern. HCP need to consider AAV patient needs at diagnosis, when assessing response to treatment, and when considering therapy initiation and changes.

![EMJ Nephrology [Supplement 4] 2020 Feature Image](https://www.emjreviews.com/wp-content/uploads/2020/10/EMJ-Nephrology-Supplement-2-2020-Feature-Image-940x563.jpg)






