Abstract
Glomerular diseases are a common cause of chronic kidney disease in several low-to-middle-income countries (LMIC). Additionally, they represent up to 52% of patients with end-stage renal disease (ESRD) in Africa. Current guideline recommendations for the treatment of glomerular diseases may not always be applicable in LMIC due to various challenges related to disease diagnosis and the availability of medicines. A treatment approach that starts with disease diagnosis and proper use of adjuvant therapies mainly targeted at blood pressure and proteinuria reduction is an effective therapeutic option and is recommended for patients in LMIC with glomerular pathologies. The use of immunosuppressive therapies in adults with glomerular diseases should, as far as is possible, be guided by the histological diagnosis obtained through renal biopsy. Prednisone and cyclophosphamide still form the bulk of treatment for glomerular diseases in most countries. Due to the adverse effects associated with immunosuppression, prednisone and cyclophosphamide use must be carefully weighed against the risk of potential side effects, and there is a need for frequent monitoring to assess treatment efficacy, patient response, and adverse effects. It is not advisable to use immunosuppressive drugs (e.g., cyclosporine) that require monitoring of plasma levels in centres where such facilities are not available, given the possible associated nephrotoxicity. The purpose of this narrative review is to provide an update on the treatment of common glomerular diseases and to highlight simple approaches to treatment in LMIC. Knowledge of guideline recommendations on the treatment of various glomerular diseases will provide important understanding on useful therapeutic approaches.
BACKGROUND
Glomerular diseases are a significant cause of chronic kidney disease (CKD) in many low-to-middle-income countries (LMIC), and CKD has been associated with a significant socioeconomic burden in many areas, particularly LMIC.1 The impact of CKD in these countries has particularly increased given the limited, or total lack of, access to renal replacement therapies.2,3 Due to the increasing prevalence of Type 2 diabetes mellitus globally, diabetic nephropathy has become a major player in the epidemiology of CKD and end-stage renal disease (ESRD).4 However, in most developing countries, including those in Asia, Africa, and Latin America, glomerular diseases remain a major cause of CKD and of incident dialysis patients.5-7 In various instances, the cause of CKD remains unknown and thus the condition is labelled as CKD of unknown aetiology.
Data from 12 African countries showed that glomerular diseases were responsible for 10.2–52.0% of patients diagnosed with ESRD in adults and children (Morocco: 10.2%; South Africa: 52.0%).8 Furthermore, data from a large Chinese study9 that reviewed 65,074 dialysis patients from 27 provinces showed the main causes of ESRD were glomerulonephritis (GN) (45%), diabetes (19%), hypertension (13%), polycystic kidney disease (2%), and others or unknown (20%). An assessment of children with CKD in Guatemala showed that diagnosis with glomerular disease was associated with a significant risk of progression to ESRD in comparison with other diagnoses (hazard ratio: 4.84; 95% confidence interval: 1.31–17.91; p=0.02), underlining the importance of GN as a cause of CKD in this population.10 Even within developed countries, glomerular diseases tend to play a major role in the epidemiology of kidney diseases among the poorer or minority groups.11
The recent Global Kidney Health Atlas (GKHA)12 publication by the International Society of Nephrology (ISN) showed that use of guidelines in treating kidney diseases is particularly reduced in low-income countries. This suggests that use of guidelines, such as those of the Kidney Diseases Improving Global Outcomes (KDIGO),13 for treating glomerular diseases in LMIC may also be low. The purpose of this review is to provide an update on the treatment of common glomerular diseases and to highlight simple approaches to treatment in LMIC.
EPIDEMIOLOGY OF GLOMERULONEPHRITIS IN LOW-TO-MIDDLE-INCOME COUNTRIES
Table 1 provides a summary of the frequency of common patterns of glomerular diseases seen in selected LMIC.14-18 Due mainly to a low workforce and inadequate infrastructure, renal biopsies are often unavailable in many developing countries;19,20 as a result, published reports only represent a fraction of true disease prevalence. Glomerular diseases in LMIC often present as nephrotic syndrome. Although published reports show that GN in these countries tends to be of the proliferative type, the indication that promotes the use of renal biopsies is the occurrence of nephrotic syndrome or nephrotic range proteinuria.21,22 Immunoglobulin (Ig)A nephropathy is rarely reported in African countries, but is very common in Asia.16,23 Although several LMIC have a high burden of glomerular diseases related to chronic infections, such as HIV and hepatitis B, these diseases appear to occur more commonly in countries in sub-Saharan Africa. As seen in developed countries, lupus nephritis (LN) is also the most common secondary glomerular disease seen in most LMIC.24
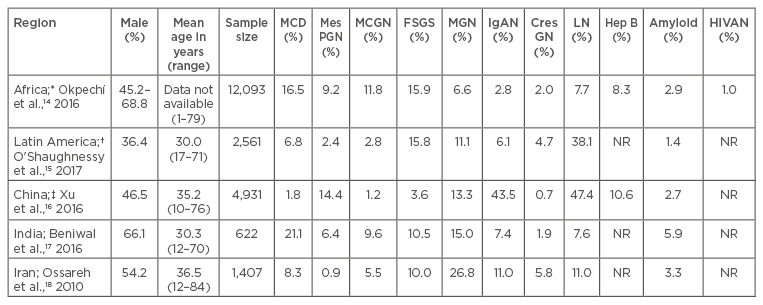
Table 1: The frequencies of primary and secondary glomerular pathologies in selected locations.
*Represents data from 13 countries: Cameroon, Democratic Republic of Congo, Egypt, Ghana, Kenya, Morocco, Namibia, Nigeria, Senegal, South Africa, Sudan, Tunisia, Zimbabwe. †Represents data from three countries: Brazil, Colombia, and Mexico. ‡Data presented separately for primary and secondary GN.
CresGN: Crescentic GN; FSGS: focal segmental glomerulosclerosis; GN: glomerulonephritis; Hep B: hepatitis B; HIVAN: human immunodeficiency virus associated nephropathy; IgAN: IgA nephropathy; LN: lupus nephritis; MCD: minimal change disease; MCGN: mesangiocapillary GN; MGN: membranous GN; MesPGN: mesangial proliferative GN; NR: not reported.
TREATMENT OPTIONS FOR GLOMERULONEPHRITIS IN LOW-TO-MIDDLE-INCOME COUNTRIES
In LMIC, glomerular diseases should be suspected early when patients present with typical clinical and urinary features, such as body swelling, rash, proteinuria, and haematuria. Knowledge of local or regional disease patterns should be used for initiating treatment, especially as renal biopsy may not be readily available. Early diagnosis and initiation of treatment could slow or halt disease progression. Figure 1 provides a summary of possible treatment options, indications for use, commonly associated complications, and the relative cost of treatment for each group of commonly occurring glomerular diseases.
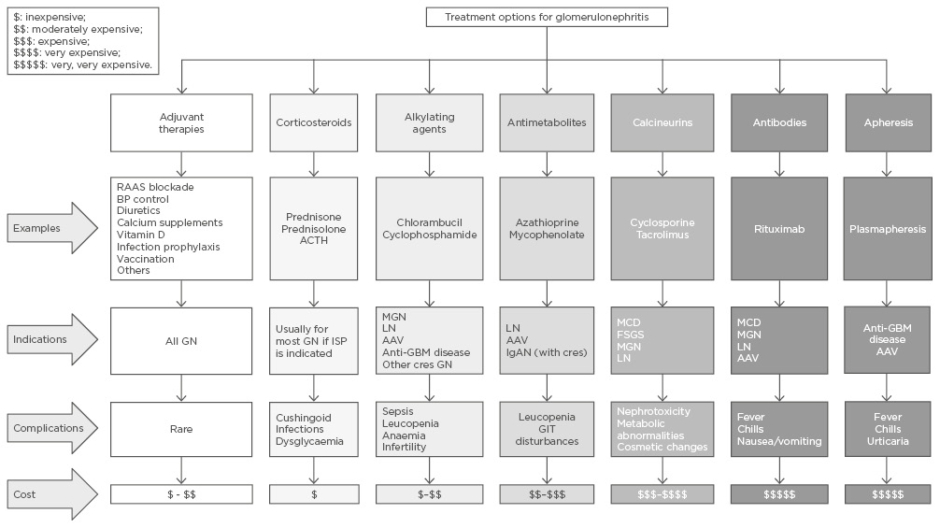
Figure 1: Treatment options for glomerulonephritis.
AAV: antineutrophil cytoplasmic autoantibody-associated vasculitis; ACTH: adrenocorticotrophic hormone; BP: blood pressure; cres: crescentic; FSGS: focal segmental glomerulosclerosis; GBM: glomerular basement membrane; GIT: gastrointestinal tract; GN: glomerulonephritis; IgAN: immunoglobulin A nephropathy; ISP: immunosuppression; LN: lupus nephritis; MCD: minimal change disease; MGN: membranous glomerulonephritis; RAAS: renin angiotensin aldosterone system.
General Treatment Measures
Adjuvant therapies form an important part of treatment of glomerular diseases. The KDIGO GN guidelines recommend measures that are directed towards control of hypertension, proteinuria, dyslipidaemia, oedema, hypercoagulable state, and increased risk of infection.13 The use of renin angiotensin aldosterone system blockade (angiotensin-converting enzyme inhibitors or angiotensin receptor blockers) is associated with a reduction in proteinuria and better control of blood pressure. Renin angiotensin aldosterone system blockade should be prescribed to all patients with nephrotic syndrome. Several studies have shown that hypertension is a predictor of poor renal outcome in patients with glomerular diseases and blood pressure control is protective against the cardiovascular risks of hypertension and delays progressive loss of glomerular filtration rate.25-27 A study28 from Brazil that evaluated the combined effects of ethnicity and hypertension in the evolution of creatinine levels between Caucasians and people of African descent showed that black patients experienced worse prognosis of renal function, probably because of hypertension and not ethnicity (p=0.027). Other general therapeutic measures employed depend on the type of disease, extent of other features of nephrotic syndrome (e.g., dyslipidaemia, presence of thrombosis), and whether immunosuppression will be used for treatment of disease. For instance, chloroquine has been shown to be useful in patients with LN and should be given to all patients who can tolerate it. One study29 from Cape Town, South Africa, showed that patients with Class V LN who were treated with chloroquine had a significantly better renal outcome than those who were never treated with chloroquine. LUMINA data also highlighted that patients treated with hydroxychloroquine showed a reduced frequency of disease activity, a lower frequency of proliferative LN, and received lower glucocorticoid doses than patients who were not treated with hydroxychloroquine.30 Bone protection with calcium and vitamin D supplements is recommended for those who will receive long-term treatment with corticosteroids to prevent osteoporosis and fractures. Infection prophylaxis against tuberculosis with isoniazid is not universally used but can be useful in areas with a high risk of such infections. Prophylaxis against other antimicrobials may be dependent on local prevalence and the pattern of disease. Other general measures include use of diuretics for oedema, vaccination to prevent infections, and anticoagulation for those at a high risk of venous thrombosis. Treatment of the specific cause of glomerular disease is important to reduce further damage to the kidneys (e.g., the use of antiviral agents for hepatitis B and C and HIV infection).
Corticosteroids
Corticosteroids, such as prednisone, form the backbone of treatment of glomerular diseases where indicated.13 Corticosteroids may be used alone in conditions such as minimal change disease (MCD) and mesangial proliferative GN (non-IgA subtype), and in patients with idiopathic focal segmental glomerulosclerosis (FSGS) if there is a good early response and achievement of early complete remission. Corticosteroids are recommended for use in combination with other immunotherapies (e.g., alkylating agents or calcineurin inhibitors) if early complete remission is not achieved in patients with MCD, FSGS, membranous GN, and in other proliferative forms of glomerular diseases. Although trialling steroid therapy without biopsy is not recommended in adult nephrotic syndrome, steroids may be given to patients who present with this condition in centres with no option for renal biopsy.
Important adverse effects of steroid therapy are often related to cumulative dose used (e.g., cushingoid appearance, osteoporosis, infections, weight gain, diabetes, or psychosis), and clinicians should be aware of these effects and frequently monitor their patients who are treated with steroids. Although steroids should be tapered during treatment, there is no consensus regarding how tapering should be carried out. KDIGO recommends to “taper slowly over a period of 6 months” for most of the conditions requiring steroid use.13 In the authors’ experience, some clinicians will taper by 5 mg every 2 weeks and others by 10 mg every month. The optimal timing of steroid discontinuation is not clear in patients with glomerular diseases; however, steroids should be discontinued early in those with resistant nephrotic syndrome to minimise adverse effects. Thus, there is a continuous need for biochemical monitoring of a patient’s response to treatment with regular assessments of protein-to-creatinine ratio in the urine. Also, for patients with steroid-dependent nephrotic syndrome, alternative treatment options should be considered early to avoid the risk of adverse effects of steroids. In centres where renal biopsy is not readily available, a trial of corticosteroids may be useful in those presenting with nephrotic syndrome, especially in children. One study31 of Indian children with frequently relapsing nephrotic syndrome reported that long-term therapy with a small daily dose of prednisolone can significantly reduce the number of relapses in patients, and that the beneficial effect may continue even after its stoppage. It is important for patients on long-term treatment to have regular assessments of their blood pressure, blood glucose, and bone scans to monitor side effects of treatment. A low threshold should be maintained for infection monitoring (blood count; X-rays; and sputum, blood, and urine cultures), and antibiotic treatment should not be delayed and should be guided by sensitivity results for those with infections.
Alkylating Agents
Due to their relatively low cost, alkylating agents, such as chlorambucil and cyclophosphamide, are readily available for the treatment of some glomerular diseases in LMIC. A recent systematic review on standard of care of patients with LN in Africa found the combination of prednisone and cyclophosphamide to be the most common therapy.32 However, given the high prevalence of infectious diseases in many LMIC (e.g., HIV, hepatitis B and C, and tuberculosis), the benefits of using an alkylating agent in any patient must always be weighed against the potential risks for infection-related adverse events. Studies from South Africa have often reported the high prevalence of infection-related complications in those treated with cyclophosphamide, with one study reporting up to 37.5% of deaths to be related to sepsis in all patients treated with cyclophosphamide.25,29 Side effects, including bone marrow suppression, infections, cystitis, and malignancies, are often related to administered dose or cumulative dose received. Trials of alkylating agents are not recommended when histological diagnosis is not available as the patient may have advanced disease. When appropriately indicated, the use of intravenous (IV) pulse dosing is a strategy that reduces the total cumulative dose of cyclophosphamide given to the patient; however, there is need to follow appropriate treatment protocols. For instance, one study33 from South Africa that looked at the outcomes of patients with idiopathic membranous GN reported poor outcomes to treatment, even though patients were not treated strictly following the Ponticelli regimen but received pulse IV cyclophosphamide. Following the study, they revised their treatment protocol for idiopathic GN to use the Ponticelli regimen, which uses daily oral cyclophosphamide. IV pulse cyclophosphamide is recommended for induction therapy in a number of diffuse proliferative GN, such as Class III and IV LN, and other forms of rapidly progressive glomerulonephritides, such as antineutrophil cytoplasmic autoantibody (ANCA)-associated GN. Although IV cyclophosphamide may be used in cases of crescentic IgA nephropathy or post-infectious GN, it is not generally recommended in non-crescentic forms of these conditions.
Daily oral cyclophosphamide is mainly recommended in adults with MCD who are intolerant of steroids or those with frequent relapsing or steroid-dependent MCD, or for those with idiopathic membranous GN or idiopathic mesangiocapillary GN (MCGN). Alkylating agents must be stopped in patients who become pregnant during therapy as they are associated with fetal malformations. Routine monitoring for side effects should include regular assessments of full blood counts and clinical assessments or surveillance for infections.
Antimetabolites
Antimetabolites (azathioprine or mycophenolate mofetil [MMF]) are often used to maintain remission in proliferative GN after induction therapy with an alkylating agent, especially in patients with LN and ANCA-associated vasculitis. However, MMF is increasingly used for induction therapy in patients with LN, although issues of cost may be a problem in some regions.32 In patients with generalised vasculitis, the withdrawal of cyclophosphamide and substitution with azathioprine after remission did not increase the rate of relapse.34 In the ALMS study,35 although MMF failed to show superiority over cyclophosphamide for induction treatment of LN (56.2% response to MMF versus 53.0% response for IV cyclophosphamide; p=0.58), MMF showed benefit in a subanalysis mainly made up of black Africans and individuals with parents of mixed ancestry (60.4% for MMF versus 38.5% for IV cyclophosphamide; p=0.033). However, MMF was superior to azathioprine for maintaining renal response and preventing relapse in patients who had a response to induction therapy.36 MMF is also recommended in adults with MCD who cannot tolerate corticosteroids, cyclophosphamide, and calcineurin inhibitors; however, antimetabolites have not been found to be effective in patients with FSGS.13,37 MMF is not recommended in patients with IgA nephropathy unless they have crescentic disease; MMF has also not shown efficacy in patients with idiopathic membranous GN and is not recommended as initial therapy or monotherapy in such patients.13,38 Although azathioprine is relatively inexpensive, the use of MMF is often limited by its cost and availability. Bone marrow suppression with increased risk of infections is a major side effect of antimetabolites. Azathioprine can also cause hepatic and pancreatic dysfunction, while MMF has been associated with significant gastrointestinal effects (e.g., bloating, nausea, and diarrhoea). In female LN patients receiving MMF, it is recommended to stop MMF and change to azathioprine if they become pregnant.
Calcineurin Inhibitors
Calcineurin inhibitors, including cyclosporine and tacrolimus, are often used as a second-line agent for treating GN in instances where patients have not responded to initial therapy or when patients are intolerant of first-line treatment. Thus, they are useful for frequently relapsing or steroid-dependent MCD, and in idiopathic FSGS and membranous GN when there are contraindications to steroid use. In patients with LN, calcineurin inhibitors are indicated in patients with Class II and in other classes for maintenance treatment in patients who cannot tolerate MMF or azathioprine, or in those with resistant disease. Multitarget therapies combining a calcineurin inhibitor (tacrolimus) and MMF have been compared with cyclophosphamide for induction therapy in a number of LN studies.39,40 One multicentre study39 from China reported more patients in the multitarget group (45.9%) than in the IV cyclophosphamide group (25.6%) reaching complete remission after 24 weeks (p<0.001); the incidence of adverse events did not significantly differ between the multitarget and IV cyclophosphamide groups (50.3% versus 52.5%). The choice of calcineurin inhibitor may be guided by cost and availability of medications. One randomised controlled study41 in children with steroid-resistant nephrotic syndrome in India showed no difference in efficacy between cyclosporine and tacrolimus when combined with low dose corticosteroids; however, the authors suggested tacrolimus as an alternative to cyclosporine in view of the lower risk of relapses and absence of cosmetic side effects.41 The cost of treatment with calcineurin inhibitors, availability of medications, and a lack of capacity for monitoring drug levels could be major issues with their usage in LMIC. Coadministration of a calcineurin inhibitor with ketoconazole could be used as a strategy for reducing the administered dose and cost. In one study,42 concomitant use of ketoconazole with a calcineurin inhibitor led to a 38% cost saving. The lack of capacity for monitoring drug levels in many countries may render this approach impractical given the nephrotoxicity associated with calcineurin inhibitors.
Monoclonal Antibodies
Due to a lack of data from randomised controlled trials, rituximab and other novel agents are usually reserved as rescue therapies in patients with disease resistant to other immunosuppressive therapies. Rituximab is recommended in patients who have failed more than one of the recommended initial regimens.13 Available data on rituximab have been largely obtained from small studies, mainly on LN and membranous GN.43-46 In many instances, the results of these studies have not been compelling enough to make strong recommendations about the use of rituximab. For instance, in the LUNAR trial,46 144 patients with Class III or IV LN were randomised to receive rituximab or placebo. The overall (complete and partial) renal response rates were not significantly different between patients receiving placebo and those treated with rituximab (45.8% versus 56.9%; p=0.18, respectively). In patients with ANCA-associated vasculitis, the RITUXVAS trial47 reported similar remission induction rates and safety between rituximab and cyclophosphamide. Similarly, in a longer-term outcomes study48 after treatment with rituximab for ANCA-associated renal vasculitis, rates of the composite outcome of death, ESRD, and relapse did not differ between the group treated with rituximab and the group with cyclophosphamide alone. The dose and treatment schedule have also varied between studies; while some studies have used 375 mg/m2 in 1–4 doses,43,47,49 others used 1,000 mg in 2–4 doses.46,50 In reality, there are only a few centres in LMIC that can afford to provide this treatment to patients because of the high cost associated with therapy and need for monitoring lymphocyte subsets (CD19+ B cells).
Plasma Exchange
Glomerular diseases requiring apheresis have not been commonly reported in LMIC (Table 1). The low reported prevalence of such conditions could be related to low incidence or lack of resources for diagnosis and subsequent treatment.51,52 Arogundade et al.52 suggested that there is a significant limitation in accessibility, availability, and use of therapeutic plasma exchange in Nigeria, and that knowledge of this treatment and its applications is minimal among nephrology professionals. Many centres are not equipped with serological assays for making a diagnosis or are unable to perform diagnostic renal biopsies. Reports from studies in LMIC often highlight poor outcomes associated with such conditions, as many patients often present late when the disease has already advanced.53-55 One trial, which assessed whether the addition of plasma exchange was more effective than IV methylprednisolone in the achievement of renal recovery in patients with ANCA-positive vasculitis and serum creatinine >500 µmol/L, reported increased renal recovery in the plasma exchange group when compared with IV methylprednisolone.56 KDIGO recommends the use of plasma exchange or plasmapheresis in patients with hepatitis C with glomerular disease and severe kidney involvement, in patients with LN with thrombotic microangiopathy, in those with ANCA-positive vasculitis with diffuse alveolar haemorrhage or renal failure, and in patients with anti-glomerular basement membrane disease.13
BARRIERS TO EFFECTIVE TREATMENT OF GLOMERULAR DISEASES IN LOW-TO-MIDDLE-INCOME COUNTRIES
Several barriers hinder the adequate treatment of glomerular diseases in LMIC. In sub-Saharan Africa, for instance, renal pathology services are scarcely available in several centres.3,20 This means patients with glomerular diseases are more likely to be treated empirically (i.e., without knowledge of the underlying pathology) with oral prednisone alone and sometimes in combination with other immunosuppressive therapies. The recently published report of the GKHA survey20 of the ISN revealed that, in several LMIC, availability of essential laboratory services, such as measurement of urine protein-to-creatinine ratio for quantifying proteinuria and measurement of serum creatinine with estimated glomerular filtration rate are often absent. Other factors, such as the cost of treatment (given that patients are often required to pay out of pocket for treatment) and availability of an adequately trained workforce, as well as availability of specific therapies, also constitute barriers to effective care for patients with glomerular diseases in LMIC.12,19
CONCLUSION
Although LMIC have the same options as developed countries for treating glomerular diseases, the frequent lack of opportunity for renal biopsy diagnosis and the high cost and unavailability of other diagnostic services, as well as the costs of some forms of therapy, constitute significant barriers towards reducing the burden of CKD associated with glomerular diseases in these regions. Efforts to improve diagnostic services for early identification of glomerular diseases, as well as early initiation of therapies, are recommended.








