Abstract
Background: Acute renal graft dysfunction (AGD) is one of the primary complications after kidney transplantation. The aim of this study was to identify the systemic oxidative DNA damage and apoptosis markers in patients with AGD, which will aid the understanding of the underlying processes of the complication.
Methods: A cross-sectional analytical study was conducted in renal transplant (RT) recipients with and without AGD. The follow-up time of patients was <1 year. Using the ELISA technique, the markers of oxidative DNA damage (8-hydroxy-2-deoxyguanosine and 8-oxoguanine-DNA-N-glycosylase-1) and apoptosis (caspase-3, caspase-8, soluble TNF receptor 1, and cytochrome C) were determined.
Results: Donor age was significantly higher in patients with AGD versus those without AGD (43±11 years versus 34.1±10.6 years, respectively; p<0.001). Levels of 8-hydroxy-2-deoxyguanosine were also significantly higher in AGD patients than those without AGD (624.1±15.3 ng/mL and 563.02± 17.4 ng/mL, respectively; p=0.039) and the DNA repair enzyme 8-oxoguanine-DNA-N-glycosylase-1 was significantly diminished in AGD patients versus non-AGD patients (7.60±1.8 ng/mL versus 8.13±1.70 ng/mL, respectively; p=0.031). A significant elevation of soluble TNF receptor levels in AGD patients was also found versus those without AGD (1178.6±25.2 ng/mL versus 142.6±39 ng/mL, respectively; p=0.03). Caspase-3 levels were higher in patients with AGD (1.19±0.21 ng/mL) versus those without AGD (0.79±0.11 ng/mL; p=0.121) and was also significantly augmented in AGD versus healthy control subjects (0.24±0.1 ng/mL; p=0.036). Cytochrome c in AGD patients was 0.32±0.09 ng/mL and 0.16±0.03 ng/mL in those without AGD versus 0.08±0.01 ng/mL in healthy controls (p=0.130 and p=0.184, respectively).
Conclusion: These findings suggest that oxidative DNA damage with insufficient DNA repair and higher levels of caspase-3 compared to controls are markers of apoptosis protein dysregulation in AGD patients.
INTRODUCTION
Renal transplantation (RT) is the best management strategy for end-stage renal disease.1 Although adequate function of the kidney is the aim of RT, diverse factors can trigger acute renal graft dysfunction (AGD): acute rejection, toxicity by calcineurin inhibitors, infections, and recurrence of the original disease.2 Graft transplantation involves an ischaemia/reperfusion process during the surgical procedure, which is continuous with pathophysiological mechanisms, such as immunosuppressive infections, and is associated with the production of free radicals with the capacity to oxidise carbohydrates, lipids, proteins, and nucleic acids.3
8-hydroxy-2-deoxyguanosine (8-OHdG) is a marker of oxidative DNA damage that increases in the presence of oxidative stress4 and reflects the magnitude of oxidative DNA damage.5,6 8-OHdG is a potentially mutagenic compound that participates as an inducer of apoptosis through activation of initiator and executor caspases.7-9 8-OHdG is therefore considered a marker of DNA damage severity.10,11 The response to DNA damage involves repair, regulation of the cell cycle, and activation of apoptosis.12 Oxidative DNA damage can be repaired primarily by the endonuclease enzyme 8-oxoguanine-DNA-N-glycosylase-1 (hOGG1).13,14
The stimulation of apoptosis can be produced in response to severe DNA damage or by severe shortening of the telomeres.15 Apoptosis involves condensation of chromatin, fragmentation of the nucleus, vesiculation of the plasma membrane, and creation of apoptotic bodies through activation of the intrinsic or extrinsic pathways.16 The extrinsic pathway is activated by death receptors belonging to the super family of TNF receptors (TNFR), which contain the death domain. The TNFR1/TNF-α or Fas/CD95 receptors are activated when they bind with ligands specific for trimerisation and transduce signals through the cytoplasmic death receptors. Interaction with TNFR1 activates the extrinsic pathway of apoptosis by activating caspase-3.17 The intrinsic mitochondrial pathway participates in the liberation of pro-apoptotic proteins from the mitochondrial intermembrane space to the cytosol (permeabilisation of the outer mitochondrial membrane).18 When severe DNA damage occurs it induces the activation of both pathways of apoptosis. In response to DNA damage, signals of phosphorylation of other proteins are activated and transduced.19 Caspase-3 is activated in the presence of oxidative DNA damage and its activation increases during dysfunction of the graft.20-22 The objective of this study was to identify systemic oxidative DNA damage and apoptosis markers in patients with AGD.
METHODS
A cross-sectional analytical study was performed in 90 patients >18 years of age who underwent RT at the Specialties Hospital of the National Occidental Medical Centre of the Mexican Social Security Institute (Hospital de Especialidades del Centro Médico Nacional de Occidente del Instituto Mexicano del Seguro Social [IMSS]), Guadalajara, Mexico; follow-up was <1 year and patients were managed with a triple immunosuppressive regimen (tacrolimus, mycophenolate mofetil, and prednisone). Two study groups were formed: 45 patients with AGD characterised by elevation of creatinine ≥30% from normal levels (average creatinine level per patient during the first post-transplant months) and 45 patients without AGD with serum creatinine <30% who attended the hospital for routine follow-up. Recipients of grafts from perished donors >55 years of age, recipients with renal comorbidities at the time of the study (blood dyscrasias or infections), patients who were undergoing a second or third RT, and patients who had ingested nonsteroidal anti-inflammatory drugs, antineoplastic drugs, or angiotensin-converting enzyme inhibitors were excluded for the study. The blood samples of 10 clinically healthy subjects (volunteer blood donors) as a healthy control (HC) group were included to establish the normal values of the reagents.
Blood Samples
A total of 5 mL of venous blood was obtained immediately prior to RT in a tube containing ethylenediaminetetraacetic acid (EDTA) and another 5 mL in a dry tube. The serum and plasma were separated at 1,800 rpm for 10 minutes and the samples were stored at -80°C until final processing.
8-hydroxy-2-deoxyguanosine
Levels of the 8-OHdG marker were determined using ELISA (ELISA kit; Abcam®, Cambridge, UK), following the manufacturer’s instructions. The samples, buffer, and standards were added to all wells except the blank. The 8-OHdG monoclonal antibody was added and the plate was incubated for 18 hours at 4°C. The plate was then washed with buffer for the recommended time and 200 µL of Ellman’s reagent was added to each well. The absorbance was read at 405 nm with the automated reader (BioTek Instruments Inc., Winooski, Vermont, USA).
Human 8-oxoguanine-glycosylase-1
Levels of the hOGG1 enzyme were determined by an ELISA Kit (Elabscience®, Wuhan, China), following the manufacturer’s instructions. The samples and standards were added to each well and the plate was incubated for 90 minutes at 37°C. The corresponding washes were performed and 100 µL of the biotinylated antibody was immediately added. The plate was incubated for 1 hour and later the enzyme conjugate and substrate were added. The optical density was read at 450 nm.
Caspase-3 and Caspase-8
Quantification of caspase-3 and caspase-8 in the serum was performed using the commercial ELISA kit (Caspase-h3 ELISA Kit and Caspase-h8 ELISA kit [MyBioSource®, San Diego, California, USA], respectively). Before performing the assay, the samples and reagents were kept at room temperature for 30 minutes. The standards and serum were added to the wells with 50 µL of the conjugate reagent and the plate was incubated at 37°C for 1 hour. The wells were washed five times and 50 µL of substrate solution A and 50 µL of substrate solution B were added, and then incubated at 37°C for 15 minutes. Then, 50 µL of stop solution was added and the absorbance was read at 450 nm.
TNF Receptor 1 Levels and Cytochrome c
The concentration of soluble TNFR1 (sTNFR1) was measured using the commercial ELISA kit (MyBioSource). Fifty microlitres of the standards and serum were pipetted into the 96-well plate and covered with the antibody, together with 100 µL of the horseradish peroxidase conjugate reagent and the resulting solution was incubated at 37°C for 1 hour. The wells were washed four times with buffer wash and 50 µL of the chromogen solution A and 50 µL of the chromogen B solution were added, and then incubated for 15 minutes at 37°C; 50 µL of the stop solution was added and the absorbance was read at 450 nm. The serum levels of cytochrome c were quantified using the commercial ELISA kit (Abcam). One hundred microlitres of serum and standards were added to the corresponding wells on the plate. Then, 50 µL of the biotinylated antibody was added and the plate was incubated for 2 hours at room temperature. The plate was washed and 100 µL of the streptavidin-horseradish peroxidase enzyme was added. The plate was incubated for 1 hour and the TMB substrate and the stop solution were added at the corresponding times. The absorbance was read at 450 nm.
Statistical Analysis
The Kolmogorov–Smirnov test was used to determine the distribution of the study variables. Variables are expressed as mean±standard deviation and in frequencies (%) according to their type. To compare biomarkers among AGD patients versus patients without AGD, and HC versus patients with AGD and without AGD, a Mann–Whitney U test was used. Chi-squared was used to compare qualitative variables. A p≤0.05 value was considered statistically significant. The data were analysed with SPSS software (v20, SPSS Inc., Chicago, Illinois, USA).
Ethical Considerations
This study was performed in agreement with the norms established by the Declaration of Helsinki (64th General Assembly, Fortaleza, Brazil in October 2013) and the Best Clinical Practices Guide (International Conference on Harmonization for Research in Human Beings). Signatures were obtained for informed consent and the confidentiality of the patients was maintained. The study was approved by the Research and Ethics Committee of the Mexican Social Security Institute (R-2015-1301-83) and by the Research Registry in the State of Jalisco (59/E-JAL/2015).
RESULTS
Clinical and Demographic Data
There were no differences in the demographic data between the study groups except for sex frequency: more RT (84%) were performed in males, and females presented with significantly less AGD (p=0.007). The average age of patients with AGD was 25.7±6 years (range: 17–52 years) and 28.0±9 years (range: 18–58 years) without AGD. As expected, the levels of creatinine were significantly higher in AGD patients (p<0.001) versus those without AGD since it was the variable used in making the groups. The levels of baseline creatinine were significantly increased in AGD patients (p=0.007) versus those without AGD. The donor age was significantly higher in AGD patients versus those without AGD (43.0±11.0 years versus 34.1±10.6 years; p<0.001) (Table 1).
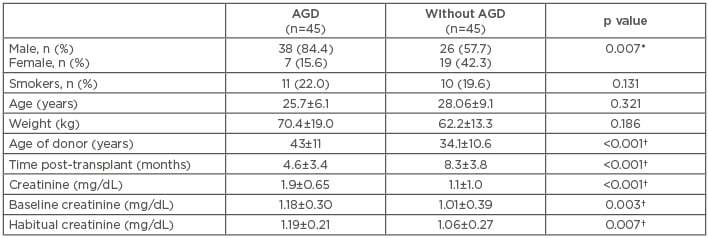
Table 1: Demographic and clinical features of the study population.
*p≤0.05, chi-squared test was used to compare frequencies between groups; †p≤0.05, for comparison of quantitative variables using a Mann–Whitney U test.
AGD: acute renal graft dysfunction.
Oxidative DNA Damage and DNA Repair Enzyme
The level of the 8-OHdG marker of oxidative DNA damage in the HC was 427.03±32.2 ng/mL, while the average was found to be significantly elevated in AGD patients (624.1±15.3 ng/mL; p=0.005). 8-OHdG levels were also increased in patients without AGD (563.02±17.4 ng/mL; p=0.063) versus HC and were significantly increased (p=0.039) in AGD patients compared to non-AGD patients. Levels of the hOGG1 enzyme in HC were 12.23±0.30 ng/mL, while the enzyme levels in AGD and non AGD patients were significantly diminished (7.60±1.8 ng/mL and 8.13±1.70 ng/mL, respectively; p<0.001) compared to HC and between AGD patients and those without AGD (p=0.031) (Table 2 andFigure 1).
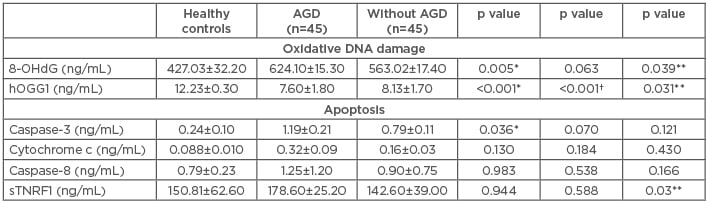
Table 2: Oxidative DNA damage and apoptosis markers.
*AGD versus healthy controls; †without AGD versus healthy controls; **AGD versus without AGD.
8-OHdG: 8-hydroxy-2-deoxyguanosine; AGD: acute renal graft dysfunction; hOGG1: 8-oxoguanine-DNA-N-glycosylase-1; sTNFR1: soluble TNF receptor 1.
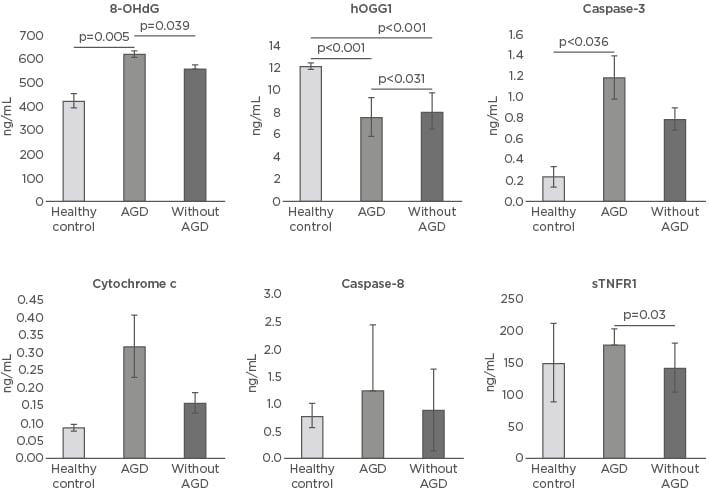
Figure 1: Oxidative DNA damage and apoptosis markers in healthy controls and patients with and without acute renal graft dysfunction.
8-OHdG: 8-hydroxy-2-deoxyguanosine; AGD: acute renal graft dysfunction; hOGG1: 8-oxoguanine-DNA- N-glycosylase-1; sTNFR1: soluble TNF receptor 1.
Caspase-3, Caspase-8, Cytochrome c, and Soluble TNF Receptor 1
The levels of caspase-3 were increased in AGD patients (1.19±0.21 ng/mL) versus those without AGD (0.79±0.11 ng/mL; p=0.121). Levels of caspase-3 were also significantly augmented in AGD patients versus HC (0.24±0.1 ng/mL; p=0.036). Levels of caspase-8 in HC were 0.79±0.23 ng/mL, 1.25±1.2 ng/mL in AGD patients, and 0.90±0.75 ng/mL in those without AGD (p=0.166). The levels of cytochrome c in HC were 0.08±0.01 ng/mL, 0.32±0.09 ng/mL in AGD patients, and 0.16±0.03 ng/mL without AGD (p=0.130 and p=0.184, respectively). The sTNFR1 levels were 150.81±62.6 ng/mL in HC, similar to patients with AGD (178.6±25.2 ng/mL) and patients without AGD (142.6±39 ng/mL) (p=0.944 and p=0.588, respectively). Nevertheless, when comparing the sTNFR1 levels between the AGD patients and without AGD, the plasma levels were significantly greater in AGD patients (p=0.03) (Table 2 and Figure 1).
Correlation Between Clinical Parameters and Apoptotic Markers
The association between the study variables and clinical parameters was evaluated. Donor age was an important factor in AGD patients, with a positive correlation to serum creatinine levels (Rho=0.36; p<0.001) and caspase-3 levels (Rho=0.29; p=0.01). There was a significant correlation between serum creatinine levels and sTNFR1 levels (Rho=0.25; p=0.017), and a positive association between the levels of serum creatinine and baseline creatinine levels (Rho=0.27; p=0.01).
DISCUSSION
The demographic characteristics of the patients included in this study demonstrate the important relationship that exists between donor age and AGD, in agreement with a previously published paper that indicates that kidneys from donors >55 years of age have a higher risk for AGD.23 In the present study, donors >55 years of age were not included but it is noteworthy that as donor age increased it was related to an increase in serum creatinine levels in recipients; this result is consistent with other published reports.24 Despite the fact that the effect of donor age on survival of the renal graft is well known, older donors are still used due to the scarcity of organs.25 The levels of caspase-3 positively correlated with donor age; however, previous reports have demonstrated that caspase-3 levels increase in the process of damage and acute kidney graft rejection, but in our results patients were acute rejection-free and higher levels of caspase-3 were found in AGD subjects compared to HC, as well as a trend to those without AGD. In the case of caspase-8, no differences were found, which suggests higher protein apoptosis in AGD.26 In 2015, Lorente et al.27 reported the novel finding of an association between serum caspase-3 levels and mortality in patients with cranium trauma. Another study has suggested a novel mechanism of caspase-8 involvement in promoting inflammation via the release of exosomes by cancer cells that contain lysyl-tRNA synthetase to induce the migration of macrophages and secretion of TNF-α.28
The results of this study revealed a significant increase in the levels of the 8-OHdG marker in patients with AGD and 8-OHdG was found to be higher in acute graft rejection patients. Matsumoto et al.29 concluded that 8-OHdG can be considered a prognostic biomarker of the graft,29 and the increase in 8-OHdG in end-stage renal disease patients subjected to haemodialysis, peritoneal dialysis, or acute renal graft rejection has been reported.30
The results of this study are also consistent with those that have been previously described since the study found that patients with AGD present more oxidative DNA damage, possibly mediated by a significant decrease in the levels of the repair enzyme hOGG1. This finding relates to a report by Xu et al.,31 who described an increase in oxidative DNA damage mediated by a decrease in its repair.
Other markers of apoptosis, such as cytochrome c liberated by the cellular mitochondria in apoptosis, are considered a key factor in the activation of the intrinsic pathway of apoptosis. This study discovered increased levels of cytochrome c in AGD patients, relating to reports from the experimental study by Tanaka et al.,32 which demonstrated that the regulation of the anti-apoptotic BCL-2 proteins diminish the liberation of cytochrome c and that this decrease favours graft maintenance. Other studies have highlighted the important role that TNF-α plays in graft rejection, and the TNF-α receptors are widely implicated in the function of this cytokine. Previous reports have suggested the relevant role of the TNFR in the development of acute rejection in RT.33,34 In this paper, the serum levels of sTNFR1 were elevated in AGD patients and the increase found correlated positively with the clinical data of renal graft prognosis. The systemic expression of oxidative DNA damage and repair and some apoptotic markers could be potential biomarkers for the follow-up of patients with AGD.
CONCLUSION
This study provides one of the first sets of evidence showing a strong association between oxidative stress, apoptosis, and acute graft dysfunction in a RT setting. Despite this, the cross-sectional nature of the study is its major limitation because causality cannot be determined; however, this study paves the way for other researchers to explore this topic. In conclusion, these findings suggest that AGD patients experience oxidative DNA damage with insufficient DNA repair, possibly mediated by apoptosis dysregulation.








