Abstract
Background: Immunisation remains critical in prevention of serious COVID-19 infection. This study aimed to characterise the prevalence of humoral and cellular immunity in patients on maintenance dialysis in a nephrology centre 8 months after vaccination onset.
Methods: Real-world single-centre prevalence cross-sectional study enrolling patients on peritoneal and haemodialysis. Humoral response was measured as specific IgG (anti-spike protein receptor-binding domain IgG) and cellular response as T cell reactivity through interferon γ quantification as response to antigen.
Results: Of the 86 patients enrolled, 79.4% and 84.1% showed humoral and cellular immunity, respectively. Anti-spike protein receptor-binding domain IgG correlated with specific T cell reactivity (ρ=0.58; p<0.001). Vaccinated patients with associated high comorbidity burden and low serum albumin were at risk of absent immunity (p<0.05).
Conclusion: The prevalence of humoral and cellular immunity against severe acute respiratory syndrome coronavirus 2 in vaccinated Portuguese patients on maintenance dialysis is high. High comorbidity burden and low serum albumin are risk factors for absent immune response.
Key Points
1. Humoral and cellular quantitative responses to vaccination correlated throughout the study of patients receiving dialysis, suggesting interdependence of the adaptative immune system.2. High comorbidity burden, quantified through Charlson Comorbidity Index (CCI), correlated with low immunity yield from vaccination in patients receiving dialysis.
3. Adapting isolation and vaccination policies to protect patients receiving dialysis who have high frailty and comorbidities scores is an important factor for a successful vaccination campaign.
INTRODUCTION
Global immunisation against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the standard-of-care in preventing COVID-19 and, in the absence of a specific antiviral therapy, the only effective action against this pandemic. Different vaccines with different action mechanisms have been developed, namely BNT162b2 (Pfizer–BioNTech [New York City, USA, and Mainz, Germany, respectively])1 and mRNA-1273 (Moderna [Cambridge, Massachusetts]),2 which are mRNA-based vaccines, as well as ChAdOx1 nCov-19 (AstraZeneca [Cambridge, UK])3 and Ad26.COV2.S (Janssen Pharmaceuticals [Beerse, Belgium]),4 which are recombinant adenovirus vectors encoding the SARS-CoV-2 spike glycoprotein.
From the start, the need for mandatory regular contact with health care services, coupled with worse disease severity and increased mortality risk, established patients on maintenance dialysis (MDP) as a high-risk population.5-7 In this setting, international recommendations, as well as local healthcare authorities, considered immunisation of MDPs a priority, starting the vaccination campaign in February 2021 in Portugal.
As time elapsed, the understanding that the inherent dynamism of a dialysis centre, with a permanent inflow and outflow of chronic patients, has led to a heterogenous dialysis population regarding contact with SARS-CoV-2 and vaccination schemes, or time of inoculation (namely before dialysis initiation, where vaccines unapproved by the Portuguese Dictectorate-General of Health for MDPs were administered). Thus, to rely on a one-time vaccination campaign is insufficient, and follow-up measures for incident patients on dialysis are required.
Aggravating this, end-stage kidney disease (ESKD) associates with immune dysfunction, affecting both the innate and adaptative system.8,9 Uremic toxins, malnutrition, chronic inflammation, and dialysis technique contribute to this impairment.10 One of the most important examples is the antigen-presenting dendritic cell, necessary to start antibody production, presenting both a quantitative reduction, while also being dysfunctional in ESKD. It is now proposed as one of the main mechanisms of immunodeficiency in this population.10-16 Additionally, antigen-specific memory CD4 T cell, responsible for lasting immunity, is also functionally defective and, on a molecular level, dysregulation of toll-like receptors and up-regulation of inflammatory cytokines all contribute to immune system stunning.17-20 These limitations have raised concerns about the immunogenicity of COVID-19 vaccination in MDPs and on the subsequent preservation of that acquired immunity.
The study aims, primarily, were to quantify the prevalence of humoral and cellular immunity against SARS-CoV-2 in a vaccinated Portuguese MDP cohort, with patients on peritoneal dialysis (PD) and haemodialysis (HD), 8 months after the first vaccination campaign and, secondarily, to compare humoral and cellular responses against clinical and demographic risk factors in the appropriate subgroups.
METHODS
The authors conducted a cross-sectional observational study of all the MDPs in a Portuguese National Health System’s medium-sized nephrology department on specific SARS-CoV-2 T reactive cell response and anti-spike protein receptor-binding domain IgG (IgG S-RBD) titres. This work followed the ethical principles presented in the declaration of Helsinki and informed consent was obtained from every participant in this study. Exclusion criteria were restricted to those who could not provide informed consent.
Blood samples were collected in October 2021 as part of the centre’s contingency protocol. Variables, including age, sex, comorbidity burden as measured by Charlson Comorbidity Index (CCI), type of vaccine, dialysis modality, presence of COVID-19 infection in the past, chronic kidney (CKD) staging at vaccination, and analytical results, which included intact parathormone (iPTH), serum albumin (sALB) and C reactive protein (CRP) being used as variables to assess for differences in vaccination response.
Quantification of Humoral Response
Measurement of immunogenicity was performed at the hospital’s clinical pathology laboratory. Quantitative determination of SARS-CoV-2 IgG (S-RBD IgG) in the patient’s serum was performed by chemiluminescence immunoassay (Maglumi [Snibe, China]), in addition to IgM anti-spike and anti-nucleocapsid for tracking past virus contact. Results were measured as AU/mL. Response was considered significant for values over 1 AU/mL, in accordance with manufacturer specifications.
Quantification of Cellular Response
For determination of the activity of SARS-CoV-2-reactive T cells, the EUROIMMUN (Lübeck, Germany) Quan-T-Cell ELISA was used, an interferon (IFN) γ released assay (IGRA) based test. Heparinised whole blood was incubated into three stimulation tubes: BLANK, no T cell stimulation, for determination of the individual IFN-γ background; TUBE, specific T cell stimulation using antigens based on the SARS-CoV-2 spike protein; and STIM, unspecific T cell stimulation by means of a mitogen, for control of the stimulation ability. The obtained plasma was analysed by ELISA and the SARS-CoV-2 specific IFN-γ-release assay was quantified automatically, in mIU/mL. The IFN-γ concentration of the TUBE after BLANK subtraction was evaluated in order to obtain information on a past pathogen contact with SARS-CoV-2, or an immune reaction following vaccination. In accordance with the manufacturer’s recommendations, concentrations between 100–200 mIU/mL were considered borderline, with under 100 mIU/mL being negative and over 200 mIU/mL positive.
Statistical Analysis
Statistical analysis was carried out using Microsoft (Redmond, Washington, USA) Excel 2016 and IBM (Armonk, New York, USA) SPSS Statistics 25 software.
Descriptive analysis was performed using means with standard deviation for continuous variables (median with interquartile range [IQR] for skewed distribution), and categorical variables using absolute and relative frequencies. For comparative analysis, specific statistical tests were performed based upon the nature of the variables: continuous/continuous–correlation with Spearman for skewed and Pearson for parametric variables; binomial/continuous–differences in median with Mann–Whitney U for skewed distribution and means with Student’s t-test, if parametric; and binomial/binomial–Fisher’s exact test and Phi coefficient, if significant.
Variables that were significantly different between immune and non-immune subgroups, cellular or humoral, were pooled together and binary regression analysis was performed to assess their contribution to the likelihood of absent immunity.
RESULTS
A total of 88 patients were enrolled, with 86 getting screened for immune response, 65 (75.6 %) patients from HD and 21 (24.4 %) from PD. Patients who refused vaccination (2) were excluded after initial recruitment. Descriptive analysis, including results from demographic, clinical, and immunity related variables, is summarised in Table 1.
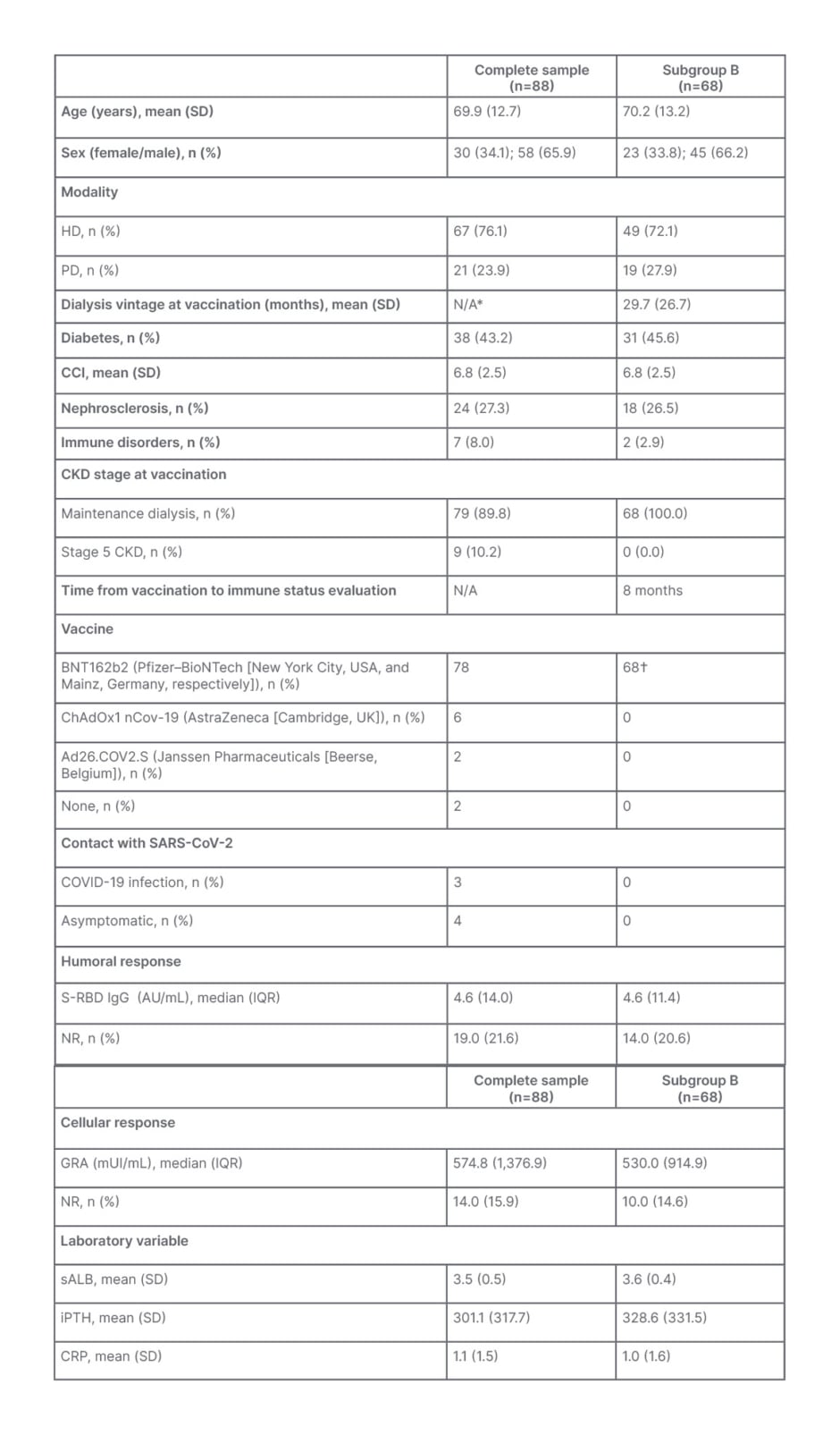
Table 1: Descriptive group and subgroup analysis.
*Both groups included patients who were not on dialysis.
†Administered at the same time.
CCI: Charlson Comorbidity Index; CKD: chronic kidney disease; CRP: C-reactive protein; HD: haemodialysis; IGRA: interferon γ release assay; S-RBD IgG: anti-spike protein receptor-binding domain IgG; IQR: interquartile range; iPTH: intact parathormone; N/A: not applicable; NR: non-responsive; PD: peritoneal dialysis; sALB: serum albumin; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; SD: standard deviation.
The group’s mean age was 69.6 years (standard deviation [SD]: 12.8), with 30 female patients (34.9%). CCI mean was 6.7 (SD: 2.5), and 39 (45.3%) had diabetes. Regarding manufacturer, 78 (90.7%) patients received the BNT162b2 (Pfizer–BioNTech) vaccine, 6 (7.0%) the ChAdOx1 nCov-19 (AstraZeneca), and 2 (2.3%) the Ad26.COV2.S (Janssen Pharmaceuticals). Time and CKD stage at vaccination differed, with 68 patients receiving vaccination in February, integrated in initial MDP vaccination protocols, while the remaining 18 received it later, with nine still on Stage 5 CKD before dialysis initiation (10.5%) and the remaining nine after dialysis start. Mean time to immune evaluation in these 18 patients was 4.4 months (SD: 1.9). A total of seven patients (8%) had a history of COVID-19 infection confirmed via PCR of a nasopharyngeal swab.
Regarding immune response, humoral response, through IgG S-RBD quantification, showed a median of 5.6 AU/mL (IQR: 14.4 AU/mL), with 19 patients (22.1%) not achieving humoral response cut-off; and cellular immunity, as quantified through T cell reactivity, revealed a median of 598.7 mUI/mL (IQR: 1,440.4 mUI/mL), with 14 patients (16.3%) showing no reactivity (<100 mUI/mL) and 9 (10.5 %) resulting in inconclusive with intermediate results (100–200 mUI/mL). IgG S-RBD levels correlated positively with IFN-γ-release assay results (ρ=0.56; p<0.001). A total of 12 patients (14.0%) were concomitantly negative for humoral and cellular immunity.
Patients with an history of contact with SARS-CoV-2 showed the highest values of specific IgG (median: 476.7 AU/mL versus 4.0 AU/mL), as well as T cell reactivity (median: 2,440.6 mUI/mL versus 695.4 mUI/mL), though none of these differences were statistically different (p=0.07 and p=0.33, respectively). Receiving the vaccine before dialysis start (in non-dialysis ESKD [N=9]) was associated with no cellular response when compared with vaccination in patients who were already on dialysis (Phi=0.44; p=0.006), which was not verified for humoral response (p=0.4).
Subgroup Analysis
A total of 68 patients (77.3% of the original sample) satisfied the selection criteria, having received 2 doses of BNT162b2 at vaccination campaign onset (February 2021), while already being on dialysis and with no clinical or laboratory findings compatible with virus contact. Mean age was 70.2 (SD: 13.2), 33.8% female, and 72.1% were on HD versus 27.9% on PD. In these patients, immunogenicity analysis, dating approximately 34 weeks from vaccination, revealed a serologic median of 4.6 AU/mL (IQR: 11.4 AU/mL) and cellular response median of 530 mIU/mL (IQR: 914.9 AU/mL). Quantitative humoral and cellular response correlated positively (p=0.5; p<0.001). Humoral and cellular responses were negative in 20.6% and 14.7%, respectively. Compared with humoral responders, non-responders presented higher CCI (8.6 versus 6.4; p=0.005), lower sALB (3.4 versus 3.7; p=0.03), and lower iPTH (95.4 versus 241.9; p=0.03), while CRP and age alone were not significantly different. On the cellular dimension, non-responders showed higher CCI (9.4 versus 6.2; p<0.001) and lower sALB values (3.2 versus 3.7, p<0.001).
When comparing complete absence of immune response in these patients (humoral and cellular concomitantly; verified in nine patients) with the remaining subgroup, even those who just achieved response in one type of the adaptative immunity, complete non-responders had higher CCI (9.4 versus 6.3; p=0.001) and lower albumin (3.2 versus 3.7; p=0.003). A binary logistic regression was performed to ascertain the effects of CCI and sALB on the likelihood that patients had no humoral and cellular response concomitantly. The model was statistically significant (□2[2]=24.1; p<0.001) and explained 62.0% (Nagelkerke R2) of the variance in non-responding while also correctly classifying 91.2% of cases. Higher levels of CCI (odds ratio: 1.9; 95% confidence interval: 1.1–3.4; p=0.03) increased the likelihood of complete absence of immune response whereas higher sALB decreased it (odds ratio: 0.02; 95% confidence interval: 0.02–0.20; p=0.01).
Further exploratory analysis was performed using the subgroup B, but specifically for differences in dialysis modalities. Patients on PD were significantly younger (61.1 versus 73.8 years; p<0.001), with lower CCI (5.4 versus 7.4; p=0.005) and higher iPTH levels (550.8 versus 242.4; p<0.001). CRP (0.7 versus 1.1; p=0.4) and sALB (3.5 versus 3.6; p=0.3) levels and were not significantly different. Regarding immune response, specific IgG titres were higher in the PD subgroup (median: 6.3 versus 3.0 AU/mL; p=0.36) and a lower rate of IgG under 1 AU/mL (10.5% versus 26.1%; p=0.1), although neither were statistically significant. T cell reactivity quantification values were statistically higher among patients on HD (median: 297.1 mUI/mL versus 695.4 mUI/mL; p=0.03), but there was no difference in the rate of cellular response under the 100 mUI/mL cut-off (20.0% versus 17.9%; p=1).
DISCUSSION
This study showed a high prevalence of humoral (78.9%) and cellular (83.7%) immunity 8 months after vaccination campaign onset in Portuguese MDPs, regardless of the time of vaccination, manufacturer, or previous contact with the virus. Humoral and cellular quantitative responses correlated throughout the study, suggesting interdependence of the adaptative immune system instead of two separate dimensions. The prevalence of patients with known contact with SARS-CoV-2 was low (8%) and does not translate the true incidence during this 8-month period, given the high mortality of COVID-19 in patients on dialysis.5-7 Similarly, interpretation of the results must consider that patients without response to vaccination were at risk of death by COVID-19 since vaccination campaign onset, which was not quantified in this cross-sectional study. Previous COVID-19 infection elicited the highest values of humoral and cellular response, though was not significantly different from non-infected, supporting the main limitation throughout all comparative analysis in this study: small sample size.
Vaccination in patients with ESKD who were pre-dialysis was associated with absence of cellular response (p=0.006), even though the time from vaccination to the immune status evaluation was shorter when compared with MDPs vaccinated in February. It is relevant to note that these patients were not exclusively vaccinated with BNT162b2 but also with ChAdOx1 nCov-19 and Ad26.CoV2.S, whereas those already on MDP were all given the BNT162b2, which was the only vaccine approved for patients on dialysis in Portugal.
Higher and untreated levels of uraemia, persistent volume overload with resulting gastrointestinal endotoxemia, decreased clearance of proinflammatory cytokines, and oxidative stress can set up the substrate for increasing circulating inflammatory cytokines and the resulting T cell dysfunction.21-23 However, even after dialysis is initiated, the presence of a vascular/peritoneal access or contact with extracorporeal components still contributes to chronic inflammation.
Moreover, studies regarding hepatitis B vaccination have suggested a decrease in immune response proportional to the degree of kidney failure, with patients on dialysis yielding the worst immunogenicity,24,25 establishing that even small levels of residual kidney function ameliorate inflammatory status in MDP.22,26 Therefore, the association between pre-dialysis ESKD, and absence of cellular immunogenicity verified in this study, could result from other factors beyond those related to CKD. Despite the limitations already mentioned, this finding of an improved response in patients on dialysis when compared with imminent pre-dialysis ESKD has not yet been reported, and may support a heavy contribution of uraemia and an exacerbated inflammatory state in immune system dysfunction.
Concerning vaccination response and immunogenicity, multiple studies have looked at early humoral response in MDP. One of the largest by Stumpf et al.,27 with a cohort of 1,256 patients on dialysis, described a rate of seroconversion of over 95% and cellular response of 78% after 8 weeks of first vaccination, establishing its efficacy in this population. A more recent study by Sibbel et al.28 provided real-world evidence of its effectiveness in lowering death and hospitalisation among MDPs. However, early follow-up of elicited immunogenicity quickly showed a steeper decline compared to healthy controls, leading to the conclusion of a shorter longevity of immune protection in MDP.29-34
In this cohort, subgroup analysis focused on this dimension, given the obvious confounding factors of utilising the complete sample (different times of vaccination, different manufacturers, and patients, with elicited immune response by direct contact with the virus). From this perspective, comparative analysis was performed only after grouping patients to attain a homogeneous group, improving the ability to address factors differentiating immune status and, so, the authors aimed to evaluate specific response to BNT162b2 and factors relating to the waning of its immunogenicity in MDPs after 8 months, since it constituted the sole stimulus (two separate doses in February 2021) for immunogenicity in this subgroup.
The results point to absent immunity of one in five patients for humoral and one in seven for cellular response. Again, these results are prevalence based and do not consider vaccinated patients who died during this period. Hence, the true value of immunity waning is probably higher. The relevance of the subgroup analysis, however, is not only to establish the prevalence of immunity but to assess for risk factors, an important and interesting addition in this part of the study. Here, and against several previous reported studies, age alone did not contribute to the lack of immunity. Instead, high CCI, comprising not only age but several disorders, including cardiovascular and connective tissue diseases, was systematically associated with overall lower immune assessed response. In a similar fashion, low sALB was also associated with this outcome, and both risk factors contributed independently to the likelihood of absence of both humoral and cellular elicited immunity.
The present study can be a step forward in the understanding and management of this and the subsequent pandemics, proposing the use of CCI and sALB as surrogate markers and valuable tools to predict lower response to vaccination and faster waning of immunity. Again, new studies with larger sample size or meta-analysis are required to establish this relation.
Dialysis modality also contributes to immune impairment differently. PD specific factors include intra-abdominal catheter; high glucose/glucose degradation products or endotoxins on dialysate; constitutive complement activation; and repetitive peritonitis and exit-site infection.21,22,35 For the population on HD, the factors include central venous catheter as vascular access; the use of conventional HD over haemodiafiltration; the use of bioincompatible dialysis membranes; and complement activation during session secondary to loss of inhibitory molecules.36-38 Regardless of this knowledge, there is still no evidence on which modality associates with the lowest immune dysfunction.
In subgroup analysis C, comparison between vaccinated patients on PD and HD in February was remarkable for lower levels. A lower rate of humoral immunity was seen in the HD subgroup, with one in four not achieving 1 AU/mL compared with one in 10 in the PD subgroup, even though the difference was not significant.
As previously discussed, comorbidity burden and sALB correlated with immune response. The first was significantly lower in PD, which can be responsible for this immunity difference. On the cellular side, however, the difference abates in rate of reaching cellular responsiveness threshold, and is even quantitively lower in the PD subgroup, this time significantly. Advances in immunology understanding have differentiated subtypes of T cells into central memory, mainly localised to the lymph nodes and those that lack immediate effector function, and effector memory T cells (TEM), peripherally localised and responsible, after stimulation, for immediate production of IFN-γ, which enhances antigen-specific adaptive immune response and is the response quantified with IGRA. Roberts et al.39 have studied the peritoneal effluent and established not only the existence of highly specialised resident TEM population in the peritoneal cavity of patients on PD (a first line of defence against pathogens), but also the selective recruitment of TEM cells from peripheral blood, including those produced through vaccination, to the peritoneal cavity. Consequently, the fact that IGRA was measured in peripheral blood samples may lead to falsely reduced values, a direct result of the constant recruitment of TEM cells to the peritoneum. Other studies, not restricted to TEM and immediate IFN- γ, but focusing on thymic epithelial cells are important to further understand long-standing cellular immunity in patients on PD.
Despite the limitations of the authors’ study, mainly related to sample size, there are many important and interesting new findings such as dialysis centres maintain high rates of immunity after 8 months of vaccination; humoral and cellular quantification correlate positively; vaccination in immediate pre-dialysis ESKD is suggested as yielding worst immunogenicity compared with MDP; high comorbidity burden and low sALB are independent risk factors for low acquired immunity in MDPs and can be used as predicting markers of patients that will show deficient immunogenicity yield; and patients on PD show a relative reduction in cellular response when using IGRA as a quantifying method, which favours the specific TEM abnormalities verified in this specific modality.
CONCLUSION
This study supports a high prevalence of both humoral and cellular immunity against SARS-CoV-2 among real-world vaccinated Portuguese dialysis centres, even 8 months after vaccination campaign onset. Moreover, the intertwining of both adaptative immunity dimensions, humoral and cellular, is highlighted through multiple correlations.
High comorbidity burden, and specifically CCI as a quantifying tool, is suggested as a surrogate marker to predict lower response or faster waning immunity after vaccination. In a holistic approach, other markers of frailty like low serum albumin may also play a role in the creation of a risk stratification panel to identify possible non-responders and those at risk of faster immunity waning, particularly when direct immune response assays are unavailable.
Taken together, these results suggest the need to adapt protocols based not only on vaccination status, but also on patients’ individual risk of no-response and of faster waning immunity. Vaccination remains the single most important measure in COVID-19 prevention, requiring that new incident patients on dialysis be procured and vaccinated to maintain high immunity rates across institutions.







