Abstract
Women who conceive while receiving peritoneal dialysis (PD) are at a high risk of encountering maternal and fetal complications. Although the occurrence of successful pregnancies in women with end-stage renal disease undergoing PD is becoming more common with advancing dialysis technology, women in this population must be monitored by a team of dedicated renal physicians and obstetric teams to ensure the best maternal and fetal outcomes are achieved. Given the haemodynamic advantages of PD over haemodialysis in pregnancy, PD therapy is the favoured renal replacement option in pregnant women with end-stage renal disease. This is particularly true when PD is initiated after conception or if pregnancy occurs within 1 year of starting PD. The management of anaemia, hypertension, dry weight adjustment, and dialysis regimen in a pregnant PD patient will undergo continuous adjustment to maintain haemodynamic and physiologic stability to meet the demands of the pregnancy-associated changes. In this article, the incidence and management of fetal and maternal complications and pregnancy outcomes in women receiving PD are reviewed based on the latest literature available.
INTRODUCTION
While transplantation provides the best pregnancy outcomes for pregnant patients with kidney disease, dialysis during pregnancy is now a viable option for those who anticipate difficulty receiving a renal transplantation. The first successful full-term pregnancy in an end-stage renal disease (ESRD) patient on haemodialysis was first reported in 1971 by Confortini et al.1 Some 12 years later, the first sustained pregnancy where the mother received peritoneal dialysis (PD) was reported in a patient who had been receiving the treatment for 2.5 years. The pregnancy was sustained until 30 weeks, but a stillborn infant was delivered, albeit after a spontaneous labour.2 Despite the many challenges faced by pregnant ESRD women, the rate of successful pregnancy and live birth has increased from 50% in the 1990s3 to near 80% in recent years,4 once the patient has successfully conceived. From the 54 reported cases of pregnant women receiving PD available in the literature since 1983, 47 cases (87%) have resulted in a successful pregnancy, but only 6 cases were full-term deliveries (Table 1).2,5-36 The improved pregnancy outcomes are interlinked with adequate residual urine, conception during peri-initiation of the PD period, medication adjustment, tailoring PD prescription, blood pressure control, and correction of metabolic and nutrition profiles.
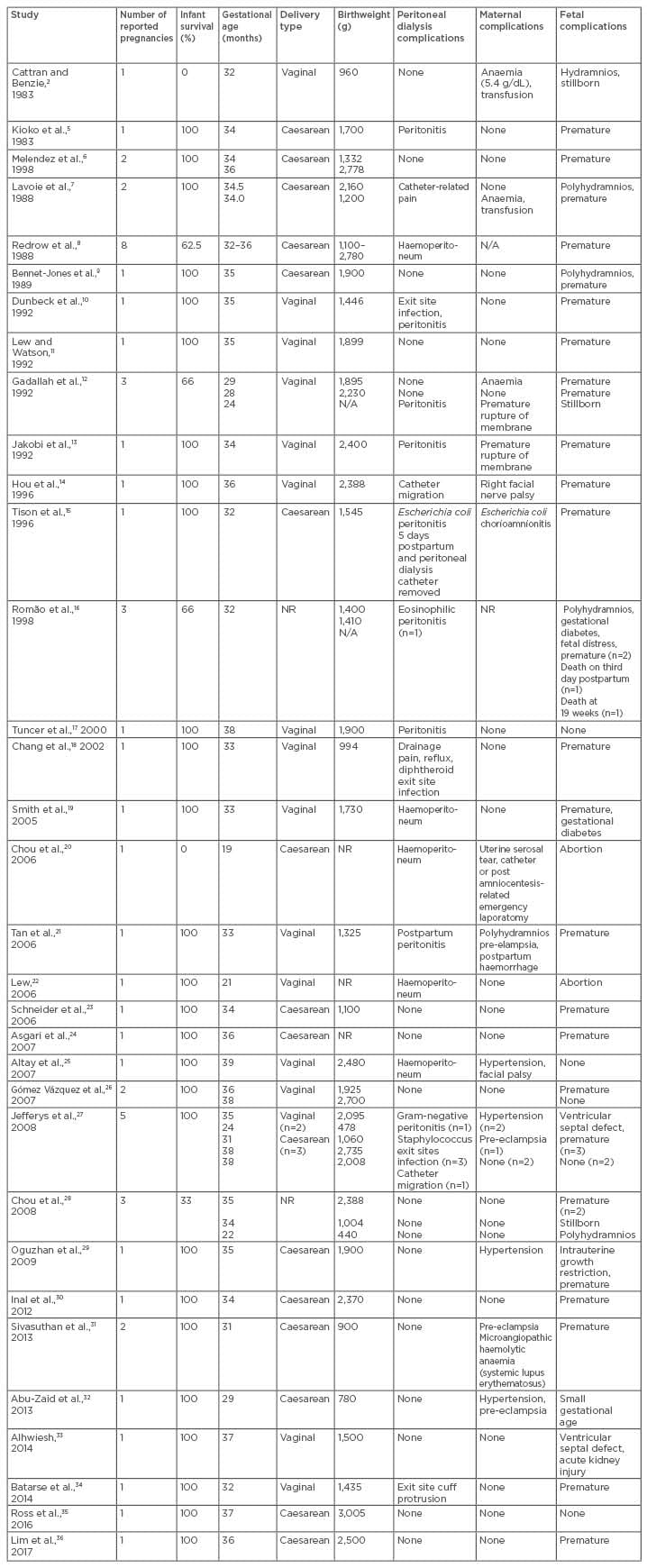
Table 1: Reported case series of pregnant women on peritoneal dialysis.
N/A: not available; NR: not recorded.
EPIDEMIOLOGY
Pregnancy in women with ESRD has always been challenging, not only for the mother but also for the newborn and the attending specialists. Therefore, the achievement of pregnancy may come as a surprise for women on PD because most have irregular menstruation, or even amenorrhea due to anovulation, making a period of amenorrhea a condition they are accustomed to. Moreover, early symptoms of pregnancy, such as early morning nausea and vomiting, are examples of, and similar to, the symptoms of uraemia or abdominal distension from PD dialysate. Achievement of pregnancy is further compounded by a failure in the surge of both luteinising hormone and follicle-stimulating hormone and low progesterone during the menstruation phase. In addition, hyperprolactinaemia is common and seen in >70% of women on dialysis. Coupled with reduced libido, it is not surprising that the conception rate in ESRD women on regular dialysis is low, reported to be in the region of 0.3–4.1%.4,37,38
Over time, improved outcomes of pregnancies in ESRD women have been increasing. The successful pregnancy rate varied from 23%, as reported by the European Dialysis and Transplant Association (EDTA),39 to as high as 70% as reported in previous case series.16,40 An evidence-based analysis of pregnant ESRD women on haemodialysis carried out from 2000–2008 showed that the overall possibility of a pregnancy resulting in living offspring is encouraging, in the range of 50–100%.41 Based on data from the Australia and New Zealand Dialysis and Transplant Registry (ANZDATA), conception on haemodialysis is approximately twice as likely as on PD.42 In another large survey of pregnancy and ESRD from the USA, 1.1% of reproductive-aged women receiving PD conceived versus 2.4% on haemodialysis.43
This lower conception rate in PD women has been postulated to be related to the presence of fluid in the abdominal cavity or inadequate dialysis intensity.42 Another hypothesis is that hypertonic dextrose solutions and the fluid-filled peritoneum interfere with ovum transit to the uterus.3 However, once conception was successful, infant survival was not significantly different between the haemodialysis and PD patients. It is also clear from the literature that the outcome of pregnancy was better in women who conceived before starting dialysis than in women who conceived after starting dialysis.5,7,13,16,26,27,30,43 Fourteen patients conceived before starting PD and all but three had successful pregnancies.2,16 There were also reported cases of using PD as a bridging therapy for optimising the renal profile of chronic kidney disease (CKD) patients during pregnancy; PD treatment was discontinued post-delivery, with patients remaining dialysis free for 2–4 years.27 A recent case report has also shown a successful, near full-term delivery in a geriatric multigravida (aged 42 years) who was receiving continuous ambulatory PD.36 In total, there have been 47 successful pregnancies from 54 pregnant women on PD during 1983–2017. These cases are summarised in Table 1.
RENAL EFFECTS OF PREGNANCY
During pregnancy, the kidneys undergo pronounced haemodynamic, structural, and endocrine changes as part of the physiological adaptation to ensure a successful pregnancy outcome. These adaptations occur as early as 6 weeks after conception and include the dilatation of the urinary collecting system, a decrease in systemic vascular resistance, and an increased glomerular filtration rate, as well as a corresponding drop in serum creatinine. During a healthy pregnancy, the kidneys will also increase the production of erythropoietin, active vitamin D, and renin. Various hormones controlling vessel tone, such as nitric oxide, endothelin, and mediators of the renin-angiotensin-aldosterone system, will also undergo adjustment. However, ESRD women are less able to make these unique renal adaptations, and this inability often leads to various clinical features characterised by normochromic normocytic anaemia, reduced expansion of plasma volume, and vitamin D deficiency. Furthermore, up to one-third of patients may experience a progressive deterioration in renal function.3,44 The risk of an irreversible loss of renal function is further magnified when the mother has uncontrolled hypertension and proteinuria.44
IMPORTANCE OF RESIDUAL URINE
Over the last decade, the literature has stressed the importance of residual urine output with regard to successful pregnancy outcomes.45 From the 54 pregnancies documented, 27 patients had good residual urine (defined as >500 mL per day) during pregnancy and all except three patients experienced a successful delivery (Table 1).2,5-36 The ADEMEX trial46 showed that residual urine can predict survival in PD populations. More recently, the presence of residual urine has been shown to be more efficient in removing uraemic toxins and protein-bound solutes compared to increasing PD prescription.47 The progressive reduction in peritoneal space in the presence of the gravid uterus in the last trimester means that the surface for adequate peritoneal exchange is insufficient and the catheter is subjected to mechanical pressure. This concern can be overcome by performing multiple frequent, but smaller, dwells. Thus, PD patients should be encouraged to try to achieve pregnancy early in the course of their treatment because it is hoped that survival benefits can be extrapolated to the products of gestation.
CONFIRMATION OF PREGNANCY
The confirmation of pregnancy in ESRD patients is challenging due to the chronically increased serum levels of beta-human chorionic gonadotropin (hCG) even in the absence of pregnancy. Apart from in placenta tissue, beta-hCG is produced in small amounts by all cells and is dependent on the kidney for a substantial proportion of beta-hCG excretion. As a result, it is not surprising that the average time taken to confirm pregnancy in an ESRD patient is 16.5 weeks.1 Therefore, among women suspected of being pregnant who have elevated serum beta-hCG, ultrasonography should be performed to verify the presence of a viable fetus and obtain the approximate gestational age.
PREGNANCY COMPLICATIONS IN MOTHERS RECEIVING PERITONEAL DIALYSIS
Women on dialysis have at least a two-fold increased risk of developing adverse maternal outcomes, including gestational hypertension, pre-eclampsia, eclampsia, and maternal mortality.45 Sonographic assessment of uterine artery blood flow at 20–24 weeks gestation can refine the risk of later pre-eclampsia and fetal growth restriction and should be used as part of the standard care. If pre-eclampsia develops, maternal renal function often deteriorates further, which can sacrifice the residual urine of the PD patients.
There are also complications that are specific to PD women: eight episodes of peritonitis and five episodes of exit site infection have been reported in pregnant women (Table 1). The postpartum period also presents an increased risk of peritonitis, during which two of the eight episodes occurred. Peritonitis was associated with premature rupture of the membrane, postpartum haemorrhage, and chorioamnionitis. Although one neonatal death has been reported,12 and another case in which the PD catheter had to be removed,15 the remainder of the cases were successfully treated. Other complications that are unique to PD therapy include haematoperitoneum,8,19,20,22 catheter malposition,14,27 catheter-related pain,7,18 and flow issues. Uterine trauma from the PD catheter remains a distinctly possible complication.20
Although maternal hypertension is associated with at least half of the cases of obstetric haemorrhage and abruptio placentae, such complications are rarely reported. Only 7 of the 53 pregnancies were documented to have maternal hypertension, which constituted a serious under-reporting. Maternal death is uncommon and has been reported to occur in 1.5% (2 out of 135 pregnancies) in a registry study of pregnant dialysis patients in the USA.43
Immunological phenomena, such as haemolysis, elevated liver enzymes, and low platelet (HELLP) syndrome; microangiopathic haemolytic anaemia; thrombotic thrombocytopenic purpura; and haemolytic uremic syndrome are more commonly reported in pregnant women with acute kidney injury than those with ESRD.48 In fact, there is a lack of reported literature regarding their occurrence in pregnant women on PD. There was only one case of microangiopathic haemolytic anaemia that occurred during the postpartum period in a patient with systemic lupus erythromatosis.31
COMPLICATIONS OF PERITONEAL DIALYSIS TO THE FETUS
A total of 42 out of 47 successful pregnancies were preterm births (Table 1). Other common fetal complications reported were polyhydramnios (n=6), intrauterine death (n=4), stillborn (n=3), gestational diabetes (n=2), fetal distress (n=2), intrauterine growth retardation (n=1), neonatal mortality (n=1), ventricular septal defects (n=2), and neonatal acute kidney injury (n=1).2,5-36 A recent meta-analysis by Piccoli et al.49 noted a significantly higher rate of small-for-gestational-age babies born to mothers on PD compared to babies born to mothers on haemodialysis; however, there was no evidence of an increased risk of congenital abnormality.
PERITONEAL DIALYSIS TREATMENT CHARACTERISTICS
While haemodialysis enables precise fluid control and, to a certain extent, adjustable clearance, it may lead to marked haemodynamic fluctuation that will disturb the placental blood flow. PD, on the other hand, provides a more stable metabolic milieu via its continuous mode of dialysis and can better preserve residual renal function. The stable intrauterine metabolic environment can reduce the risk of developing polyhydramnios, a risk that correlates with elevated urea levels that occur before a haemodialysis session.50 Polyhydramnios could also be caused by enhanced solute diuresis by fetal kidneys or decreased oncotic pressure as a response to the rapid fluctuation in urea level. Once polyhydramnios develops, the risk of premature labour is increased.50,51
DOSE OF PERITONEAL DIALYSIS AND UREA REDUCTION
There is not a clear guideline regarding the target clearance for pregnant PD patients. A target clearance of Kt/V in the range of 2.2–2.4 has been advocated since the early 1990s.52 More recent literature reported that Kt/V in the range of 2.2–6.0 has been achieved and, more importantly, these clearances are in part supplemented by a respectable renal clearance.15,18,19,23,25,35,36 To achieve this Kt/V target, a dialysate volume of up to 22 L per day has been suggested.15 This approach will invariably double the dialysate cost alongside requiring a PD continuous cycler machine that would further inflate the cost of treatment. A combination of haemodialysis and PD was first described by Lavoie et al.7 in 1988, whereby the patient received a standard 8 L per day of continuous ambulatory PD supplemented by haemodialysis three times a week through a subclavian catheter. This baby was successfully delivered at 34 weeks gestation through a caesarean section. In 2016, Ross et al.35 described another case of successful pregnancy, where the patient received cycler PD treatment of 8 L per day supplemented by intermittent haemodialysis for 3.0–4.5 hours, five times per week, starting at 9 weeks gestation.35 Table 2 illustrates the different regimes of PD prescriptions used in pregnant PD patients.2,5-36
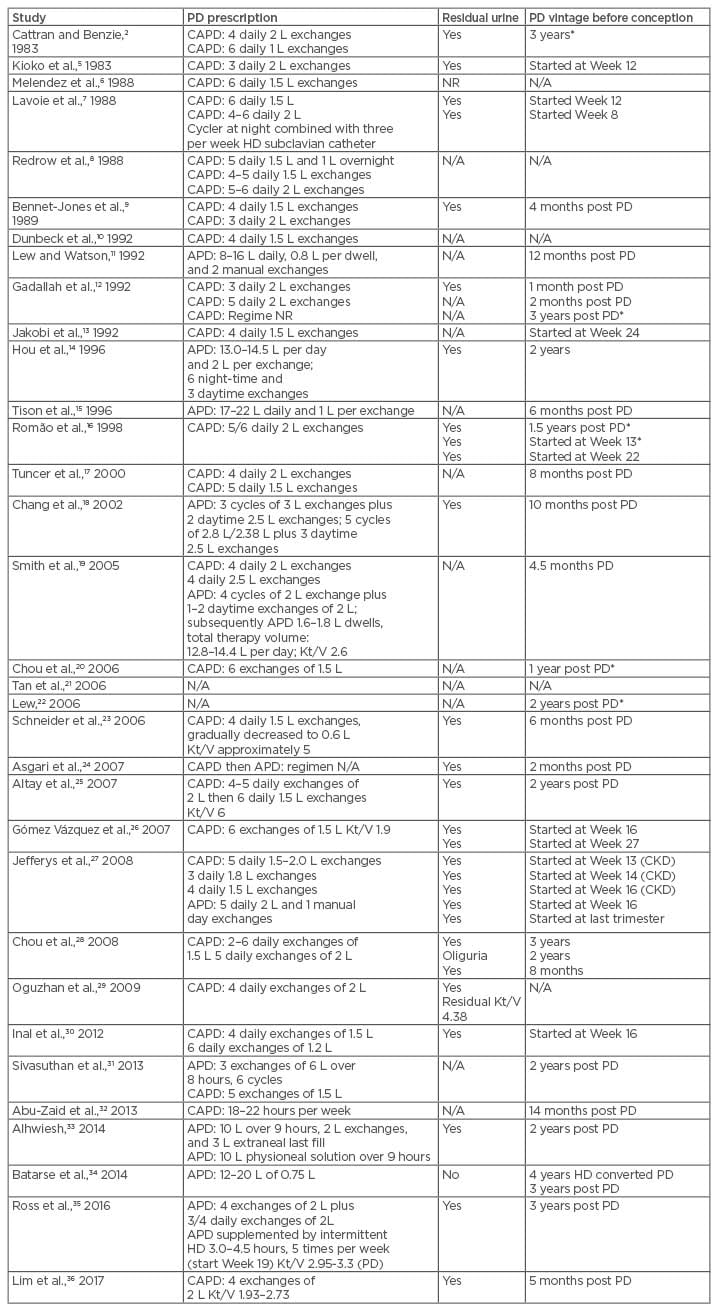
Table 2: Peritoneal dialysis vintage, prescription, and residual urine.
*Pregnancy failure.
APD: automated peritoneal dialysis; CAPD: continuous ambulatory peritoneal dialysis; CKD: chronic kidney disease; HD: haemodialysis; K: dialyser clearance of urea; N/A: not available; NR: not recorded; PD: peritoneal dialysis; t: dialysis time; V: volume of distribution of urea, approximately equal to patient’s total body water.
In continuous ambulatory PD, the PD prescription can be modified by increasing the number of exchanges rather than using larger volumes, since large volumes are not well tolerated in the last trimester. In patients receiving automated PD, the dialysis prescription should be modified with an increase in the total volume and therapy time, increasing the number of cycles and using smaller dwell volumes. In the practical sense, most nephrologists would choose to treat the patient clinically, monitoring blood parameters and adjusting the PD prescription as needed rather than following the Kt/V number; this is supported by an Italian best position paper and guidelines published in 2015.53 In the guidelines, the authors do not recommend using Kt/V and/or peritoneal creatinine clearance as a measurement of dose of dialysis in pregnancy due to the lack of studies considering these markers with respect to pregnancy outcomes. This contrasts with data derived from a haemodialysis population in which a meta-regression analysis showed an inverse relationship between hours of dialysis per week and haemodialysis, preterm delivery, and small for gestational age fetuses.53
ELECTROLYTE IMBALANCE
During the course of pregnancy, potassium levels remain normal despite an increase in serum aldosterone, perhaps due to the potassium-sparing effects of elevated progesterone.54 For ESRD women with residual urine, there will be a further risk of hypokalaemia due to vomiting during morning sickness, PD dialysate loss, and the effect of aldosterone on renal tissues.54 The total serum calcium concentration falls during pregnancy due to reduced serum albumin, but ionised calcium levels remain normal.54
ANAEMIA
Erythropoietin-stimulating agent (ESA) regulates erythropoiesis by stimulating the differentiation and proliferation of erythroid precursors and the release of reticulocytes into the circulation, as well as synthesis of cellular haemoglobin. The usual dose of ESA for a patient on PD is 50 U/kg twice weekly.53 It is worthwhile to note that although ESA is recognised by the U.S. Food and Drug Administration (FDA) as a Category C drug in pregnancy, it has been widely prescribed in pregnant CKD women without many adverse events.53 In fact, the dosage of ESA is frequently adjusted upwards by 50–100% due to increasing body weight. Iron supplementation at a dose of 1–15 mg/day and folic acid 1 mg/day enhance the efficiency of ESA and iron stores should be assessed before ESA is initiated.53 It is advisable that the patient maintains their haemoglobin levels at 10–11 g/dL, haematocrit at 30–35%, and serum ferritin of 200–300 µg/mL.53
DRY WEIGHT ADJUSTMENT
The patient’s fluid status should be reviewed by the nephrologist and obstetrician closely. Ideally a weekly or fortnightly ultrasound of the uterus should be carried out from the second trimester onwards to assess the growth and weight of the fetus. Dry weight must be reviewed continuously because the patient is expected to gain between 0.3 kg and 0.5 kg of weight per week during the second and third trimesters. The dry weight of pregnant women on dialysis can be difficult to ascertain. The patient will have to be assessed using a traditional method of examining overloaded symptoms, such as orthopnoea and shortness of breath, and signs including bibasal crepitations, bipedal oedema, and raised jugular venous pressure. The use of a bioimpedence device to assess dry weight is not recommended due to a lack of safety data in this group of patients.
BLOOD PRESSURE
Poorly controlled hypertension contributes significantly to the risk of pregnancy failure, including the risk of early pregnancy loss, superimposed placental ischaemia, and pre-eclampsia, as well as premature delivery and fetal growth restriction.55 Extrapolating the data from Control of Hypertension in Pregnancy Study (CHIPS),56 which randomised women to a diastolic blood pressure of 85 or 100 mmHg, showed that treating hypertension in pregnancy to a tighter target is not associated with adverse neonatal effects or pregnancy outcomes. As such, blood pressure should be tightly controlled with pregnancy-safe medications, such as long-acting nifedipine, labetalol, and methyldopa.57
NUTRITION
Supplementation of water-soluble vitamins and minerals, such as folic acid, is essential in early pregnancy. Other vitamins that should be supplemented are vitamin C, thiamine, riboflavin, niacin, and vitamin B6. Since malnutrition is common in pregnancies of ESRD patients, it is mandatory to avoid protein restriction in pregnant women on PD. Malnutrition is often caused by the lack of appetite experienced by pregnant women on PD due to the sugar load in dialysate and the delayed gastric emptying effect of dialysate inside the peritoneal cavity. Malnutrition can also be caused by the hypercatabolic effect of pregnancy in ESRD and the decreased appetite induced by acidosis and urea levels. Since malnutrition is a constant problem experienced by pregnant ESRD patients, attention should be paid to reaching the caloric target of 3,035 kcal/kg/day to reduce protein wasting. Individualisation of specific nutrient needs is paramount and the patient should have a dietary plan tailored by a dietitian or nutritionist. It has been estimated that the minimal daily dietary protein intake in pregnant haemodialysis patients should be 1.8 g per kg body weight/day;58 however, there has been no recommendation of dietary protein intake for pregnant PD patients. The closest recommendation is extrapolated from those PD patients who are at risk of protein depletion, for whom 1.4–2.1 g per kg body weight/day of protein is advised.59 The precise control of calcium and phosphorus metabolism may also be disturbed by pregnancy. Routine vitamin D supplementation during gestation may reduce the risk of pre-eclampsia, low birthweight, and preterm birth in the normal population.60 Vitamin D deficiency (<20 ng/mL) and insufficiency (20–29 ng/mL) are more prevalent among patients undergoing dialysis due to defective 1,25-dihydroxycholecalciferol synthesis.61 Thus, supplementation of sufficient calcium and vitamin D3 should occur to ensure patients remain in a positive calcium balance.
MEDICATIONS
Low-dose aspirin is recommended for the prevention of pre-eclampsia in pregnant patients with CKD;62 however, there is no recommendation for routinely prescribing aspirin for pregnant PD patients. Commonly used medications that are harmful to the fetus are angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers. Both medications have significant teratogenic effects if their use is not monitored and is continued beyond the first trimester.63 If the patients have autoimmune diseases or have undergone renal transplantation, the use of immunosuppressive therapy, such as cyclophosphamide, mycophenolate, methotrexate, and mTOR inhibitors is to be avoided. Phosphate binders, such as sevelamer carbonate, lanthanum carbonate, aluminium hydroxide, cinacalcet, and paricalcitol, have not been tested or established for use during pregnancy or lactation.64,65 In patients with residual renal function, potential nephrotoxic agents, such as aminoglycosides and nonsteroidal anti- inflammatory drugs, should be avoided.
DELIVERY AND POSTPARTUM CARE
Planned induction of labour at 37 weeks gestation or just beyond is routinely recommended for patients with no maternal or fetal complications. Planned induction allows clinicians to drain the dialysate, ensuring the patient is well dialysed prior to delivery. Vaginal delivery is preferred, and caesarean delivery is recommended only when there is a clear clinical indication. After a successful pregnancy, it usually takes a few months for the physiological changes of pregnancy to resolve completely. During this transition period, readjustment of the dry weight, residual urine monitoring, blood pressure, and a complete review of drug treatment, with special attention to the antihypertensive agent or ESA, are necessary. Breast milk analysis from lactating dialysis patients was similar to samples from low-risk control mothers, and therefore breastfeeding can be considered a viable option for mothers receiving dialysis.66 Similarly, breastfeeding should be encouraged in women on PD. Contrary to popular belief, ACE inhibitors are barely detectable in breast milk; captopril, enalapril, and quinapril are the preferred ACE inhibitors to use in the postpartum period because they not found in breast milk.67
CONCLUSION
Successful management of pregnant women with ESRD on PD must emphasise a comprehensive and co-ordinated approach between the nephrologist, obstetrician, primary care physicians, and the patient. Preservation of residual urine plus frequent monitoring of blood pressure, adjustment of dry weight, fetal growth, and biochemical features will enable timely expert intervention to achieve optimal pregnancy outcomes in pregnant ESRD women on PD. It remains to be seen whether active supplement with vitamin D or hybrid therapy (frequent or prolonged haemodialysis or haemofiltration to mimic renal clearance) will bring any additional positive impact on pregnancy outcome.








