Abstract
Intra-abdominal candidiasis (IAC) is the second most common form of invasive candidiasis after candidaemia. IAC is a broad term and can be classified on the basis of anatomical site (Candida peritonitis, pancreatic candidiasis, biliary tract candidiasis, gastrointestinal candidiasis, and hepatosplenic candidiasis) as well as clinical setting (community acquired versus nosocomial). The risk factors linked with IAC are candida colonisation, anastomotic leak, multiple instrumentation, long-term broad spectrum antibiotic use, total parenteral nutrition, and immunocompromised state. Clinically, IAC is not different from intraabdominal bacterial infection. Patients generally present with signs and symptoms of intra-abdominal sepsis after not responding to antibiotic therapy and with a background history of multiple surgical interventions or history of delayed source control. Radiological investigations, like ultrasonography and computed tomography scan, not only aid in diagnosis but also assist in differentiating medical from surgical cases. Microbiological diagnosis requires isolation of candida from an intra-abdominal specimen. Differentiation between colonisation and infection is difficult. Generally, progressive and persistent colonisation is associated with high risk of infection. Blood cultures have poor sensitivity for IAC. Non-culture based techniques used for diagnosis are mannan/anti-mannan assay, beta-D glucan assay, and validated polymerase chain reaction. Four types of antifungal strategies described in the literature are prophylaxis (risk factor driven), pre-emptive (colonisation or biomarker driven), empirical (fever driven), and targeted therapy (microbiology driven). Over recent years, global epidemiology has shown a shift from Candida albicans to non-albicans. Local epidemiology plays an important role in selection of the appropriate empirical therapy. The purpose of this review is to discuss different types of IAC based on their classification, risk factors, and management.
INTRODUCTION
Infections due to candida (candidiasis) can be classified as i) superficial candidiasis, which includes infection of skin and the mucous membrane; ii) locally invasive candidiasis (IC), which includes oesophageal candidiasis, Candida cystitis, etc.; and iii) IC comprising candidaemia and deep-seated candidiasis.1 IC remains a perplexing problem for physicians. Characteristically, it targets the compromised host, remains clinically undifferentiated from bacterial co-pathogens, takes significant time to grow in blood cultures, is rapidly fatal if not treated appropriately, and increases morbidity along with cost of care even if treated appropriately.2,3
IC in patients with intra-abdominal infections can present as isolated intra-abdominal candidiasis (IAC), isolated candidaemia, or IAC with concomitant candidaemia. Global epidemiology remains unclear. One of the largest point prevalence studies (EPIC II) conducted across 75 countries reported candida as the fourth most common isolate responsible for causing infection in intensive care unit (ICU) patients. In the study population, abdominal sepsis was the second most common site of infection after respiratory tract.4
Most epidemiological national surveillance and multicentre data originate from the USA and Europe. The rate of IAC has been reported as 4.7 per 1,000 admissions in one study. The largest study to date in this field, with the most robust data, was done by Bassetti et al.5 They studied 481 IAC patients from 13 countries, admitted from 2011–2013. The inclusion criteria was, as according to the guidelines, given by a multidisciplinary expert panel. However, the true incidence of IAC remains elusive due to the following reasons.
Firstly, blood cultures have poor sensitivity, as candida is rapidly cleared from the blood. Many cases of IAC remain undiagnosed because blood cultures do not detect all cases of candidaemia and tissue cultures are not always possible in patients with suspected deep-seated infection.6 Secondly, most studies either report IAC or isolated candidaemia in patients with intra-abdominal infections. There are very few studies that have reported the complete spectrum of IC in intra-abdominal infections.
Thirdly, IAC is a broad term, and it includes multiple conditions with different aetiologies (Figure 1). Currently, appropriate classification and nomenclature is lacking in the literature, resulting in scarce data in this field. Recently, in a consensus statement given by multinational experts, various agendas regarding IAC were addressed.7
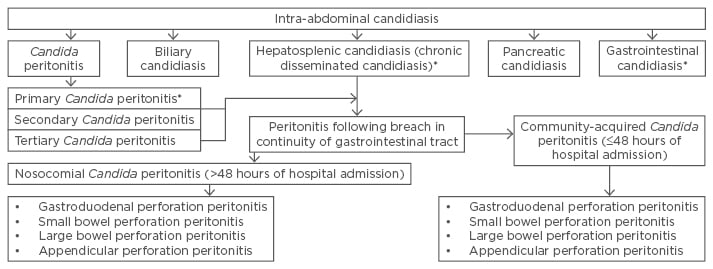
Figure 1: Classification of intra-abdominal candidiasis.
*Not discussed in the article.
Fourthly, candida is a normal flora of the gastrointestinal tract. Similar to enterococci, it has remained unclear whether its presence in an intra-abdominal specimen is relevant for therapy or outcome. Unlike candidaemia, isolation of candida in an intra-abdominal specimen is not synonymous with the need for antifungal therapy.
Treatment strategies for IAC have been classified as prophylactic, pre-emptive, empirical, and targeted. Prophylactic therapy is given to a subgroup of patients that have ≥1 risk factors for IAC. Pre-emptive therapy is based on colonisation density or biomarkers such as beta-D glucan. In this strategy, patients undergo regular surveillance of candida colonisation or biomarker serum levels. Once a predefined threshold is crossed, a patient becomes a candidate for antifungal therapy. A recently conducted randomised controlled trial (INTENSE) involving 241 patients undergoing gastrointestinal surgery for intra-abdominal infections from 53 centres across 17 countries failed to show benefit of pre-emptive antifungal therapy over placebo.8 However, the study did show that patients with a positive beta-D glucan result had a higher risk of confirmed IAC (odds ratio [OR]: 3.66; 95% confidence interval [CI]: 1.01–13.29) as compared to those with negative results.
Empirical therapy is a fever-driven approach. Fever in patients with various risk factors for IAC is used as a trigger to start antifungal therapy. Targeted therapy is the term used when antifungal therapy is given to patients with microbiologically proven IAC. Antifungals commonly used for treatment of IAC are azoles, echinocandins, and polyenes. Azoles act by interfering in fungal ergosterol synthesis and thus cause fungal cell membrane abnormalities. Polyenes (amphotericin B and its various lipid formulations) cause multiple pore formation in the fungal cell membrane while echinocandins interfere with fungal cell wall synthesis. Among the three groups, echinocandins are associated with the fewest side effects as their target site of action (i.e. the cell wall) is absent in human cells.
The current article is an attempt to describe various forms of IAC including their classification, risk factors, diagnosis, and management.
LITERATURE SEARCH
A literature search using keywords including “intra-abdominal candidiasis”, “pancreatic candidiasis”, “candida peritonitis”, and “biliary candidiasis”, from 2000–2017 was completed on PubMed, MEDLINE, EMBASE, and Google Scholar. References from relevant articles were also searched manually. Studies dealing with IAC were included in the review.
CANDIDA PERITONITIS
Definition and Classification
Peritonitis is defined as inflammation of peritoneal lining of the abdominal cavity, mostly caused by ≥1 infecting pathogen. Peritonitis occurring in patients without an obvious breach in continuity of the gastrointestinal epithelium is known as primary peritonitis.9,10 Secondary peritonitis occurs in patients with disruption of gastrointestinal continuity leading to soiling of the abdominal cavity with gastrointestinal contents. Tertiary peritonitis is a term used to describe those cases in which initial definitive treatment fails to control the peritonitis. Such patients may require multiple surgical interventions.11
Anatomical site and subtypes
Candida peritonitis can be divided into subtypes on the basis of anatomical site of perforation. Most studies show increased incidence of Candida peritonitis in patients with gastro-duodenal perforations. Appendicular perforation rarely leads to Candida peritonitis.12 In a study by Sandven et al.,13 candida was isolated from intra-abdominal specimens in 33 (30%) out of 109 patients. When patients with an appendicular perforation were removed, the percentage increased to 39.5% (32 out of 81). Another method of classification is community-acquired peritonitis and nosocomial peritonitis.14
Epidemiology and risk factors
The rate of Candida peritonitis in patients with gastrointestinal perforation varies between 30% and 48%.15-19 Studies dealing with Candida peritonitis have been summarised in Table 1. Due to the lack of a clear demarcation between candida colonisation and candida infection of peritoneal cavity, robust literature is lacking in this field.20
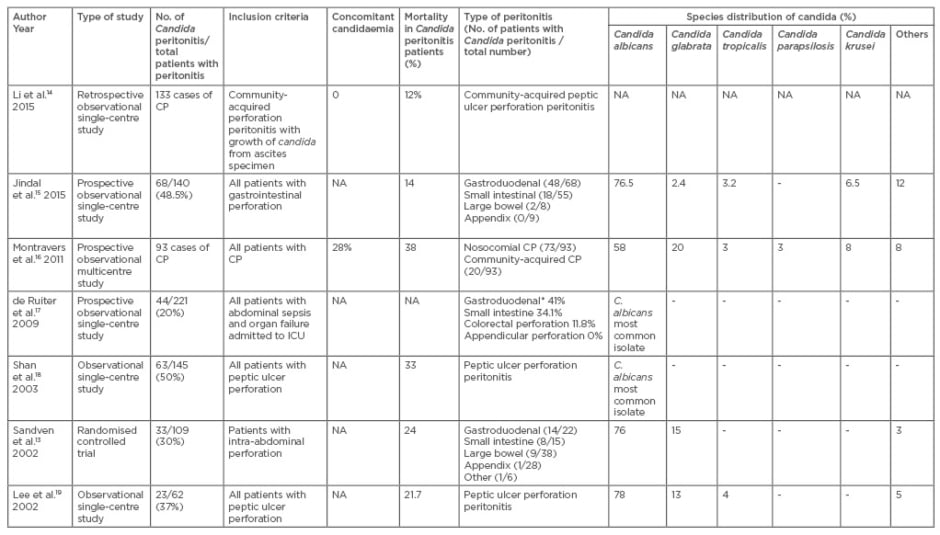
Table 1: Studies showing Candida peritonitis studies from 2001 onwards.
*Type of perforation is expressed as a percentage.
CP: Candida peritonitis; NA: not available; ICU: intensive care unit.
Species distribution
Over the past two decades there has been a shift from albicans to nonalbicans species in immunosuppressed and ICU patients.21 A species like Candida glabrate, which is the second most commonly isolated species, is less susceptible, while Candida krusei is inherently resistant to fluconazole.22 Knowledge of local epidemiology and resistance pattern can correctly guide the initial empirical antifungal therapy.23
Montravers et al.16 described different case fatality ratios for different candida species in a study including 93 patients of Candida peritonitis. The case fatality ratio was higher for Candida kefyr (4/5, 80%) as compared to Candida albicans (22/63; 35%). Case fatality ratios were 7/22 (32%), 3/9 (33%), and 1/3 (33%), for Candida glabrata, Candida krusei, and Candida tropicalis, respectively.
Candida as a risk factor for mortality
Despite the ongoing controversy between the pathogenic and non-pathogenic nature of candida, its isolation has been linked with increased risk of death in certain subgroups. Montravers et al.,24 in a case control study involving 91 cases and 168 controls of secondary and tertiary peritonitis, demonstrated isolation of candida as an independent predictor of mortality in patients with nosocomial peritonitis (infection >48 hours after admission) but not in community-acquired peritonitis (48% versus 28%). Upper gastrointestinal perforation was associated with increased risk of death (OR: 4.9; 95% CI: 1.6–14.8) in nosocomial peritonitis patients. Sandven et al.13 showed that detection of yeast in intraoperative, intra-abdominal specimen was associated with higher risk of death (OR: 11.5; p=0.03).
In a study by Dupont et al.,25 upper gastrointestinal origin for peritonitis was found to be an independent risk factor for yeast isolation in severe intra-abdominal infections. Upper gastrointestinal perforation was identified as an independent risk factor for mortality in another study by the same author involving 83 Candida peritonitis patients.26 Mortality in Candida peritonitis ranges between 12% and 38% in various studies.14-19
Pathogenesis
Its natural history involves progressive colonisation followed by invasion. In patients with peritonitis, other factors include extent and speed of debridement, adequacy of source control, number of re-operations, etc.
Calandra et al.,27 in their landmark paper, studied 49 surgical patients in whom candida was isolated from ≥1 intra-abdominal specimen. Patients were divided into Group A (19 patients), in whom candida was considered pathogenic, and Group B (30 patients), in whom candida was considered non-pathogenic. Candida was regarded as pathogenic when isolated from intra-abdominal abscess or with postoperative peritonitis. In cases of mixed bacterial and fungal peritonitis, candida was regarded as pathogenic when associated with concomitant candidaemia or non-resolving clinical condition despite appropriate surgical management and antibiotic therapy. Group A patients had significantly higher mortality as compared to Group B patients. They were also subjected to multiple reoperations due to recurrent gastrointestinal perforations as compared to Group B patients, who mostly recovered after single surgical intervention. Authors highlighted that initial heavy growth of candida or a serial rise in the amount of candida growth should be considered highly predictive of infection.
Peritoneal contamination converts to invasive disease in the background of inadequate or delayed source control in a patient on broad spectrum antibiotic therapy.28 The most important determinant of the course of illness in patients with intra-abdominal sepsis is ‘source control’.29 The adequacy of source control has not been properly addressed in most of the studies on IAC.
Diagnosis
Diagnosing fungal peritonitis is a challenge. Blood culture has low sensitivity (50%) for IC. In order to differentiate between candida colonisation and infection, an expert panel recommended systemic antifungal therapy to be considered only when the microbiological sample was obtained surgically or within 24 hours of external drainage. Positive cultures from the drains placed for >24 hours should not be treated.10
Newer diagnostic methods based on non-culture-based techniques are being employed to differentiate candida colonisation from infection.30 Beta-D glucan acts as a serum marker for early detection of invasive fungal infection.31 A recently published meta-analysis reported the sensitivity of this test as 76.8% and specificity as 85.3%.31 León et al.,33 in a study on 176 non-neutropenic patients of severe abdominal conditions, showed that beta-D glucan with a positive test for Candida albicans germ tube antibody accurately differentiated candida colonisation from IC.33
Management
Antifungal therapy in Candida peritonitis is still controversial. Antifungal prophylaxis is recommended in patients with recurrent perforation and anastomotic leaks by Canadian and European guidelines.34,35 According to Infectious Diseases Society of America (IDSA) guidelines, patients with recent abdominal surgery, an astomotic leaks, or necrotising pancreatitis should be considered for empirical antifungal therapy.36 Fluconazole achieves peritoneal concentration almost equal to that of serum after intravenous administration. Peritoneal concentrations of amphotericin B have been found to be variable. In one study, it was lower than serum level even during continuous infusion.37 Weiler et al.38 reported that though lipid formulations of amphotericin B achieve higher concentration in ascitic fluid, the concentrations were still low and may lead to treatment failure. Micafungin has been shown to have moderate penetration inside the peritoneum. Therapeutic levels were achieved for Candida parapsilosis (0.125–0.25 mg/L) and Candida albicans (minimal inhibitory concentration: 0.008–0.016) in a study including 10 patients of nosocomial peritonitis.39 Caution is required in species with lower sensitivity.
PANCREATIC CANDIDIASIS
Definition and Classification
Pancreatic candidiasis is the term used when there is microbiological isolation of candida from pancreatic tissue. Infection occurs characteristically in the middle and late phase of severe acute pancreatitis.40 The risk of infective complications increases proportionally with the extent of pancreatic necrosis. Pancreatic candida infection is termed primary when positive culture is obtained during initial intervention (radiological/ endoscopical/surgical). It is termed secondary when it occurs after prior intervention. The term ‘tertiary infection’ is used for patients with persistent inflammation and super-infection after surgery.
Epidemiology
The incidence of pancreatic candidiasis ranges between 5% and 68% in patients with severe pancreatitis, depending upon the patient population studied.41 Due to lack of standard definition and variation in diagnostic criteria, there is a wealth of data which is invalid for inter-institution comparison. Schmidt et al.42 evaluated microbial flora in a retrospective study of 78 patients with pancreatitis who underwent endoscopic transmural drainage and necrosectomy for infected walled off pancreatic necrosis. Fifty-five patients (78%) had culture proven infected necrosis, while 23 had sterile necrosis. Enterococci were the most common pathogen, responsible for 45% of the infections followed by Enterobacteriaceae (42%). Candida (22%) was the third most common pathogen. Candida was common in the antibiotic treated group (20% versus 4%, respectively). Fungi isolation at the time of index endoscopy was associated with increased risk of mortality.
According to the author’s experience, IC occurred in 8 (27%) out of 30 consecutively studied severe acute pancreatitis (SAP) patients.43 Among these eight patients, two had isolated, deep-seated infection (necrosum/drain fluid positive), three had isolated candidaemia, while three had both candidaemia and a deep seated infection. Multispecies candidiasis (>2 species) was found in two patients. Though mortality was not different between IC and the non-candidiasis group, patients with IC had increased durations of mechanical ventilation, shock, and days of ICU stay.
Hall et al.44 conducted a single-centre retrospective observational study of 101 SAP patients admitted to ICU, out of which 18 (17.8%) developed IC. Mortality in patients with invasive candida infection was significantly higher as compared to SAP patients without invasive candida infection (55.6% versus 24.1%; p=0.02). Various studies dealing with fungal infections in SAP are listed in Table 2.42-53
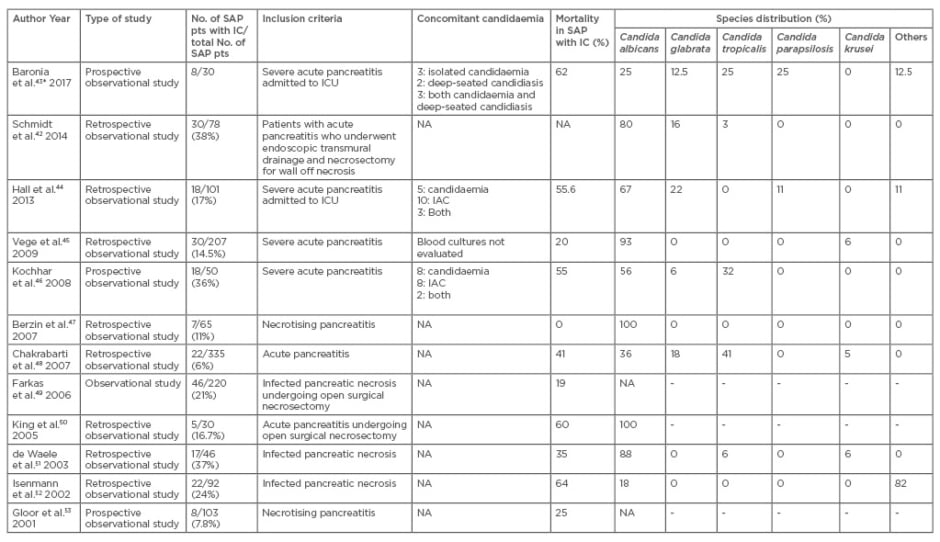
Table 2: Studies on invasive candidiasis in acute/severe acute pancreatitis from 2001 onwards.
*Species identification done only for candidaemia isolates in this study.
SAP: severe acute pancreatitis; IC: invasive candidiasis; ICU: intensive care unit; NA: not available; IAC: intra-abdominal candidiasis.
Fungal Versus Bacterial Infection in Severe Acute Pancreatitis
Vege et al.45 compared the outcome of fungal versus bacterial infection in a retrospective study involving 207 patients with SAP. Fifty-two percent of patients developed bacterial infections, while 30 (15%) also had concomitant candida infection. Candida infection was primary in 7 patients and secondary in the remaining 23 patients. Mortality rates were not different in bacterial infection and candida infection groups (20% versus 17%; p>0.41), but patients with candida infection had higher rates of organ failure and longer ICU and hospital stay.
Risk Factors
Prolonged broad spectrum antibiotic use is considered a risk factor for pancreatic fungal infection but evidence is inconclusive. Total parenteral nutrition, breach in mucosal and skin barrier due to percutaneous drainage tubes, and central venous catheters are other risk factors for IC. Candida colonisation was found to be an independent risk factor for IC in 101 SAP patients admitted to the ICU in a single-centre observational study.44
Diagnosis
The spectrum of the disease can vary from low grade infection to fulminant sepsis with multiple organ failure which remains unresponsive to antibiotic therapy. Once the diagnosis of infected pancreatic necrosis is made, necrotic material for culture and sensitivity can be obtained either through computed tomography (CT)-guided needle aspiration or during endoscopic or open necrosectomy. Blood cultures have poor sensitivity, as candida is rapidly cleared from the blood. Non-culture based techniques (beta-D glucan, mannan, and anti-mannan antibodies, polymerase chain reaction [PCR]-based assays) are increasingly being used for early and rapid diagnosis of these infections.
Management
Treatment of candida infection of acute necrotising pancreatitis involves source control as well as systemic antifungal therapy. Echinocandins are the drugs of choice for targeted as well as empirical therapy in haemodynamically unstable patients, with the exception of infection with Candida parapsilosis. Fluconazole is the preferred agent in infection with C. parapsilosis, as well as haemodynamically stable patients with no history of azole exposure. Antifungal prophylaxis remains an unresolved issue.54 There are limited reports on antifungal drug concentration in pancreatic tissue. Shrikhande et al.,55 in a study including 15 patients undergoing pancreatic surgery, reported that mean fluconazole concentration in the pancreas was 96% of the corresponding serum concentration. Penetration of the drug in pancreatic pseudocyst is slow and lower than that in plasma.56
BILIARY CANDIDIASIS
Definition
‘Biliary candidiasis’ is used when candida is isolated during microbiological analysis of bile fluid. While making a diagnosis of biliary candidiasis, it is important to differentiate infection from colonisation and contamination.
Epidemiology
There is scarcity of literature in the field of biliary candidiasis. Reports from various authors show variable rates of candida isolation from bile depending upon the cohort studied (Negm et al. 10%,57 Lenz et al. 44%58). Studies related to biliary candidiasis are listed in Table 3.58-62
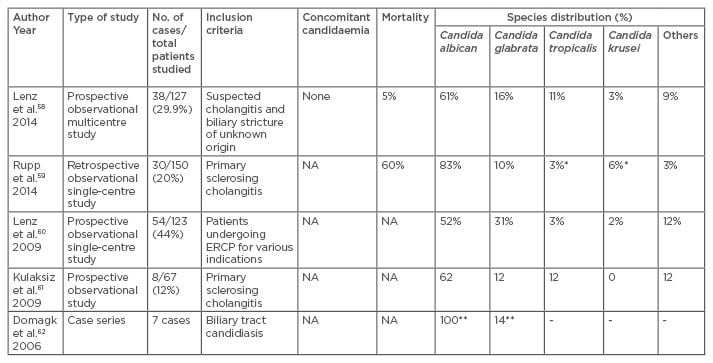
Table 3: Studies on biliary tract candidiasis from 2001 onwards.
*In one patient krusei and tropicalis were concomitantly detected, **One patient showed growth of two
species i.e. Candida albicans and Candida glabrate.
NA: not available; ERCP: endoscopic retrograde cholangiopancreatography.
Pathogenesis and Risk Factors
The pancreatobiliary system is a sterile environment. Sphincter dysfunction can play a role in translocation of candida from the gut into the relatively sterile environment of the pancreatobiliary system. In agreement with this concept, Lenz et al.58 found previous endoscopic sphincterotomy (EST) to be an independent risk factor for biliary candidiasis in a multicentre study involving 127 patients. Other factors include repeated instrumentation, stasis, immunosuppression, prolonged broad spectrum antibiotic use, and surgery.
There are two observational studies on biliary candidiasis in primary sclerosing cholangitis (PSC) patients.59,61 In one study,63 8 out of 67 PSC patients showed growth of candida in their bile samples. Patients with biliary candida had more severe cholangitis and higher CRP and serum bilirubin levels. In another study, 150 PSC patients admitted to a single centre from 2002–2012 were analysed. Thirty patients were diagnosed as biliary candidiasis. They were sub-classified as transient biliary candidiasis (15 patients) and persistent biliary candidiasis (15 patients). Patients with persistent biliary candidiasis had reduced survival with a greater need for liver transplantation.
Diagnosis
Clear diagnostic criteria are lacking in literature. Domagk et al.62 published a case series of seven patients with biliary tract candidiasis. In two patients, hypoechoic material was visible in trans-abdominal ultrasonography, while in six patients a diagnosis of biliary mycosis was made via endoscopic retrograde cholangiopancreatography, where they were able to extract obstructing fungal tissue from the biliary tract.
Lenz et al.58 suggested an algorithm for diagnosis and management of biliary candidiasis based on a multicentre prospective observational study conducted at three tertiary care centres in Germany. Bile samples from 127 patients of suspected cholangitis and biliary stricture of unknown origin were studied. The algorithm included candida antigen serology, infection parameters, and histological evidence for correct selection of patients for therapy.
Management
Treatment of biliary candidiasis includes systemic antifungals as well as endoscopic interventions (drainage of obstructed system via stent placement, debridement of biliary system by balloon catheter, placement of naso-biliary tube for local delivery of antifungal suspensions). Hepatotoxicity of the antifungal drugs should be considered when selecting an agent. Fluconazole and echinocandins are associated with lower risk of hepatic injury. Among the echinocandins, anidulafungin showed the lowest risk of elevated liver enzymes. Pooled risk estimates from randomised controlled trials showed elevation of liver enzymes (but not requiring stopping of treatment) was highest for voriconazole (19.7%), followed by itraconazole (17.4%) and amphotericin B formulations (14.1%).
CONCLUSION
IAC may include Candida peritonitis, pancreatic candidiasis, biliary candidiasis, hepatosplenic (chronic disseminated), and gastrointestinal candidiasis. True differentiation between colonisation, contamination, and infection is difficult in IAC. Patients with progressive persistent colonisation, surgery, multiple instrumentation, immunosuppression, prolonged broad spectrum antibiotic use, and multiple organ failure are at increased risk of IAC. Current data in this field are heterogeneous due to lack of uniformity in diagnostic criteria. Further research in the field can be streamlined by conducting studies as per the recommendations given by a multidisciplinary expert panel.








