Abstract
Vascular access is the main risk factor for bacteraemia, hospitalisation, and mortality among haemodialysis (HD) patients. The type of vascular access most associated with bloodstream infection is central venous catheter (CVC). The incidence of catheter-related bacteraemia ranges between 0.50 and 6.18 episodes per 1,000 catheter days and increases linearly with the duration of catheter use. Given the high prevalence of CVC use and its direct association with catheter-related bacteraemia, which adversely impacts morbidity and mortality rates and costs among HD patients, several prevention measures aimed at reducing the rates of CVC-related infections have been proposed and implemented. As a result, many clinical trials, systematic reviews, and meta-analyses have been conducted to assess the effectiveness, clinical applicability, and long-term adverse effects of such measures. An integrative review was conducted on prophylactic measures against CVC-related infections in HD patients, identifying their potential advantages and limitations. A literature search was performed within multiple databases and meta-analyses on clinical experience with prophylactic antimicrobial therapy in HD CVC were reviewed and appraised.
INTRODUCTION
Haemodialysis (HD) is the most widely used dialysis modality worldwide and requires vascular access. Access options include arteriovenous fistula (AVF), arteriovenous grafts, and central venous catheter (CVC), which can either be tunnelled or not tunnelled.1,2
Infection is still the main cause of morbidity and mortality in patients treated with HD, despite advances in preventive care and antimicrobial therapy. According to the US Renal Data System (USRDS) registry, infection is the second cause of death in patients on dialysis. Among patients with chronic kidney disease (CKD) undergoing dialysis in the USA, the total death rate is 176 per 1,000 patient-years and septicaemia accounts for approximately 26 per 1,000 patient-years.3-5
Vascular access is a major risk factor for bacteraemia, hospitalisation, and mortality among HD patients. The type of vascular access most associated with bloodstream infection (BSI) is CVC (48–73%), which also increases morbidity and mortality rates, as well as HD costs.4-7 Others infections related to catheter usage are exit site infections (ESI) and tunnel infections.
The National Kidney Foundation (NKF) Kidney Disease Outcomes Quality Initiative (KDOQI) clinical practice guidelines discourage the use of catheters as vascular access for HD and recommend that <10% of patients should be using them for access.7,8 However, the use of catheters for permanent HD access and, consequently, the number of prevalent HD patients dialysing through a CVC has progressively increased. According to the NKF, the number of prevalent patients dialysing through a catheter rose from 19% in 1998 to 27% in 2002.7 Today, >80% of incident HD patients and 18% of prevalent patients use a CVC in the USA,9,10 a reduction from 27% to 18% in prevalent patients.11 Data from the Dialysis Outcomes and Practice Patterns Study demonstrates that 18% and 34% of prevalent patients use CVC in Europe and Canada, respectively.8,12
Catheter-related bacteraemia (CRB) is the most severe CVC-related infection. CRB is defined by the Centers for Disease Control and Prevention (CDC) as bacteraemia in a patient with an intravascular catheter, with at least one positive blood culture obtained from a peripheral vein, clinical manifestations of infection (i.e., fever, chills, and/or hypotension), and no other apparent source for the infection. This can be determined through either positive semiquantitative (>15 colony-forming unit/catheter segment) or quantitative (>103 colony-forming unit/catheter segment) culture, whereby the same organism is isolated from the catheter segment and a peripheral blood sample; simultaneous quantitative cultures of blood samples with a ratio of ≥5:1 (CVC versus peripheral) and a differential period of CVC culture versus peripheral blood culture positivity of >2 hours.10
However, in recent review articles, the standards requiring peripheral blood cultures have been questioned regarding the difficulties in performing venepuncture from HD patients, the fragility of vessels, peripheral vascular disease, and the priority of preserving veins for fistula creation.10 Thus, simpler requirements, especially for epidemiological surveillance purposes, have been proposed to define CRB as positive blood cultures obtained from the catheter and blood line connected to the CVC, determining differential time to positivity.11-13
There are scarce data on epidemiology of ESI related to tunnelled CVC and most studies have focussed only on CRB.17 ESI in tunnelled CVC rate ranged from 0.35 to 8.30 episodes per 1,000 catheter days.14-17 According to Goulart et al.,14 the overall incidence of ESI was 3.50 per 1,000 catheter days. Risk factors for ESI were presence of diabetes and tunnelled CVC implanted in femoral site (relative risk [RR]: 1.56, 95% confidence interval [CI]: 1.35–1.89, and RR: 1.62, 95% CI: 1.22-1.94, respectively; both p<0.05). The most frequent agents of ESI were Gram-negative (69%), mainly Serratia marcescens, Escherichia coli, Proteus mirabilis, Pseudomonas aeruginosa, and Klebsiella pneumoniae extended-spectrum β-lactamase (ESBL)-producing. Across the time period, there was a change in aetiologic agents; Pseudomonas and ESBL agents became more frequent, while Proteus and E. coli became less frequent (p<0.05). Among Gram-positive agents, 59% were resistant to methicillin. On the other hand, Gram-negative bacilli were not often multidrug-resistant. The catheter was removed in 17% of patients due to unsuccessful treatment of ESI and was associated with Pseudomonas (p=0.04) and BSI caused by the same agent of ESI (p=0.03). Catheter survival was shorter in the ESI group (logrank: 2.92; p<0.001). These data suggest the routine application of topical antibiotic ointments to prevent ESI related to CVC caused by Gram-negative agents.17
CRB rate is highest in HD patients using a CVC and increases linearly with the duration of catheter use. The incidence of CRB ranges between 0.5–6.1 episodes per 1,000 catheter days.10,18 Several multicentre randomised studies have shown that the rate of catheter-related CRB is much higher than that of AVF-related BSI. CRB can lead to bacterial endocarditis, epidural abscess, septic arthritis, and septic embolism.4
CVC entails a risk of developing sepsis 2–5-fold higher than AVF and is therefore associated with a 25% increase in cost.10 CVC use is associated with an independent increase in mortality rate.12 Rates of mortality from infection within the first year of HD are currently 2.4-times greater than in 1981, a fact widely attributed to the use of CVC.10
In addition to the potential complications inherent to infectious processes, the rate of adverse cardiovascular events increases (up to 2-fold) after an episode of sepsis. As a result, morbidity, hospitalisation rates, and treatment costs increase, while survival rates decrease.5,10
Within the 24 hours after insertion, micro-organisms often form a biofilm in 100% of the catheters.13 Many microorganisms may adhere to the CVC surface or become incorporated within a fibrin sheath that envelopes the CVC. The adherence of organisms to the catheter surface initiates biofilm production. Biofilm is a community of organisms protected by an exopolysaccharide matrix that is stimulated and secreted by the organisms. Mature biofilms develop high resistance to systemic antibiotics requiring high concentrations for bacteria elimination.10,13,19-21
There are two main routes by which organisms enter the bloodstream to cause CRB: an extraluminal pathway and an intraluminal pathway. The extraluminal pathway involves initial contact between skin surface organisms and the external surface of the catheter at the time of CVC insertion, or before complete exit site healing and subcutaneous tunnel endothelialisation. The intraluminal pathway involves transfer of organisms by contact from the hands or skin accessing CVC tips.9,19,21
Given the high prevalence of CVC use and its direct association with CRB, which adversely impacts morbidity and mortality rates among HD patients, several prevention measures aimed at reducing the rates of CVC-related infection have been proposed and implemented.19 As a result, a large number of clinical trials, systematic reviews, and meta-analyses have been conducted to assess the effectiveness, clinical applicability, and long-term adverse effects of such measures.
This article aims to review prophylactic measures against CVC-related infections in HD patients, identifying their potential advantages and limitations. A comprehensive search was performed within Google Scholar, PubMed, and Science Direct databases from January 2008 to January 2018, using the following search terms: “hemodialysis”, “tunnelled central catheter”, “catheter-related bacteraemia”, and “prophylactic antibiotic therapy”.
PROPHYLACTIC NON-ANTIMICROBIAL MEASURES AGAINST CENTRAL VENOUS CATHETER-RELATED INFECTIONS IN HAEMODIALYSIS
The training and education of healthcare personnel in manipulating catheters regarding universal hygiene precautions has been noted as the key step toward infection prevention.14 The introduction of a catheter care protocol, which followed the guidelines published in 2002 from the CDC, resulted in a decrease in CRB incidence from 6.7 to 1.6 episodes per 1,000 catheter days.16 Top general precautions include washing hands with conventional soap and water or with alcohol-based hand rubs before and after palpating catheter insertion sites, and before dressing a CVC.19,21
The use of a sterile gown, sterile gloves, and sterile full-body drape during CVC insertion is defined as a maximum sterile barrier (MSB). In a randomised controlled trial, maximal sterile barrier precautions were compared with sterile gloves and a small drape during the placement of CVC. The MSB group had fewer episodes of both catheter colonisation and BSI (RR: 0.32; 95% CI: 0.10–0.96; p=0.04 and RR: 0.16; 95% CI: 0.02–1.30; p=0.06, respectively).19
Other measures include selection of the solutions used for exit site cleaning, dressing material, catheter antimicrobial impregnation, catheter material, topical ointments, and intraluminal compounds known as lock therapy.14,16
Superior skin and exit site cleaning have been demonstrated using chlorhexidine >0.5%. However, 70% alcohol or 10% povidone-iodine remain effective alternatives if chlorhexidine cannot be used.10,19
A meta-analysis of 4,143 catheters (1,493 CVC, 53 of which were used for HD), suggested that chlorhexidine gluconate reduced the risk for CRB by 49% (95% CI: 0.28–0.88) when compared with povidone–iodine. The absolute risk reduction was 7.1% for colonisation and 1.1% for BSI. The test for heterogeneity of treatment effect was significant for catheter colonisation (p<0.001), but not for CRB (p=0.200).17 Available evidence indicates that the use of chlorhexidine would result in a 1.60% decrease in the incidence of CRB, a 0.23% decrease in the incidence of death, and a saving of $113 per catheter.14 Recent data indicate no significant differences between transparent, semipermeable dressings and standard gauze dressings regarding CVC-related infections in HD patients. The CDC recommends the use of a chlorhexidine-impregnated sponge dressing for temporary, short-term catheters in patients >2 months of age, if CRB rate is not decreasing despite proper personnel education and training.19 Specific recommendations regarding the use of tunnelled catheters and the infection preventive measures that should be taken during their use remain unavailable and surrounded by controversy.16
The material the catheter is made from influences microbial adherence and the ability to form biofilm. Polytetrafluoroethylene or polyurethane catheters have been associated with fewer infectious complications.19 Most dialysis catheters are made of silicone or polyurethane. However, whether these materials differ in their susceptibility to biofilm formation after catheter placement has not been investigated.21
The use of catheters coated with antimicrobial agents in intensive care units is associated with reduced catheter colonisation and decreased catheter-related BSI incidence, and would therefore be a useful option for HD in patients at high risk for CRB.21-24 However, the few studies addressing the impregnation of tunnelled catheters for HD as a prophylactic measure against infections have shown conflicting results.21
MAIN STUDIES USING PROPHYLACTIC ANTIMICROBIAL IN HAEMODIALYSIS CENTRAL VENOUS CATHETERS
Topical Antibiotics
The application of topical antibiotic ointments at the CVC exit site has been shown to be associated with a 75–93% reduction in the risk of CRB. However, some agents are incompatible with some catheters, making it necessary to check manufacturers’ recommendations before applying any agents on catheters.
Mupirocin, povidone–iodine, polysporin triple antibiotic ointment (gramicidin + bacitracin + polymyxin B), and medical honey have been the most commonly studied substances.10 In 2002, Johnson et al.20 conducted a randomised trial comparing the effect of exit site application of mupirocin versus no ointment in 50 HD patients with tunnelled catheters. Mupirocin reduced the incidence of exit site infection (6.6 episodes per 1,000 catheters days versus 0 in the mupirocin group; p<0.050) and CRB (35% versus 7% in the mupirocin group; p<0.010), and also increased median bacteraemia-free survival from 55–108 days (logrank score: 7.0; p<0.010). This improved infection-free survival was explained by a reduction in staphylococcal infection log-rank: 10.7; p=0.001).25
James et al.,26 in a meta-analysis involving 15 studies with HD patients, evaluated the efficacy of topical antibiotic use or lock therapy compared to non-use of antibiotics for reduction of CRB and ESI related to the catheter. Both prophylactic antibiotic therapies reduced BSI rates and catheter withdrawal compared to non-use of prophylactic antibiotics. The antibiotic in the exit site also reduced ESI rates (0.06 versus 0.41 infection per 100 catheter days) and this reduction was not observed in studies containing lock therapy. However, in the studies analysed, several types of antibiotics and other associated interventions were used, making it difficult to analyse the individual impact of the topical antibiotic, in addition to a short follow-up period.
In 2010, Cochrane published a review on interventions to prevent CRB in HD patients. The analysis included 10 studies, totalling 786 patients; the studies evaluated interventions with topical use of mupirocin, triple polysporin, povidone-iodine, or medicinal honey, versus placebo, another antiseptic, or no topical antibiotic. They found that the use of ointment with mupirocin reduced the risk of ESI caused by S. aureus (RR: 0.18, 95% CI: 0.06–0.60; p<0.05) and CRB (RR: 0.17; 95% CI: 0.07–0.43; p<0.05) and triple polysporin ointment reduced the risk of CRB (RR: 0.4, 95% CI: 0.19–0.86; p<0.05) and all-cause mortality (RR: 0.22, 95% CI: 0.07–0.74; p<0.05), but with no effect on mortality related to infection. Povidone–iodine ointment reduced the risk of CRB (RR: 0.10; 95% CI: 0.01–0.72; p<0.05) and the use of topical honey did not significantly reduce either the risk of ESI or the risk of bacteraemia associated with a catheter when compared to mupirocin or povidone-iodine ointment.27
Since 2011, the CDC has recommended the use of ointment in the ES of the catheter after insertion and in each HD session. The use of povidone–iodine ointment or the one containing bacitracin, gramicidin, or polymyxin B is recommended, but the latter is no longer available in the USA and has never been available in Brazil. The use of ointment containing bacitracin, neomycin, or polymyxin B is cited as an option, but there are a lack of studies demonstrating efficacy in the prevention of ESI and CRB. Other options would be mupirocin or for the dressing to be impregnated with chlorhexidine. However, the CDC emphasises the risk of developing bacterial resistance, the possibility of the ointment being ineffective against the pathogens responsible for the infections, and the possible chemical interaction between the ointment ingredients and the catheter material.28,29 The 2006 Guideline of the Canadian Nephrology Society recommends the use of topical antibiotics as a form of prophylaxis.14 However, despite mentioning the use of antibiotics and the use of medicinal honey in the ES as a preventative measure of CRB associated with the catheter, since there are no studies that evaluate the development of bacterial resistance in the long term, the KDOQI guideline does not include this practice in its recommendations.4
Thus, routine use of topical antibiotics in the exit site of CVC is not widely used and should be based on the rates of local infections and the practice of each centre.30
Antimicrobial Lock Solutions
Although some studies between 2006 and 2010 have assessed the efficacy of antimicrobial lock solutions (ALS) in preventing BSI, most of them had significant limitations: these included the use of a small number of patients; many studies being retrospective, while others, despite being prospective, had short follow-up periods; some included patients with short-term and long-term catheters; and some used several solutions concomitantly, with or without antibiotics.31-33
The authors evaluated the efficacy of catheter-restricted filling using antibiotic lock solution in preventing CRB.34 A total of 233 HD patients requiring 325 new tunnelled catheters were enrolled in this study. Patients with a tunnelled catheter were assigned to receive either antibiotic–heparin lock solution (antibiotic group: cefazolin 10 mg/mL, gentamicin 5 mg/mL, and heparin 1,000 U/mL) or a heparin lock solution (no-antibiotic group: heparin 1,000 U/mL). CRB developed in 32.4% of patients in the no-antibiotic group and in 13.1% of patients in the antibiotic group. CRB rates per 1,000 catheter days were 0.57 in the antibiotic group versus 1.74 in the no-antibiotic group (p<0.0001). Kaplan–Meier analysis also showed that mean CRB-free catheter survival was significantly higher in the antibiotic group than in the no-antibiotic group log-rank: 17.62; p<0.0001). There was no statistically significant difference between the two groups in drug-resistant germs. There were statistically significant differences between the two groups in the catheter removal causes, with higher rate of infectious cause in control group (12.32 versus 2.22%; p<0.0001) and mechanical cause in ALS group (28.26 versus 37.78%; p<0.0001). The results suggest that cefazolin and gentamicin, used as antibiotic lock solution, may be beneficial in reducing the CRB rate in HD patients with a tunnelled catheter, without association with the emergence of resistant strains. However, mechanical complications were more prevalent in the antibiotic group.
Labriola et al.35 published a meta-analysis in 2007 that included eight randomised studies (829 patients, 882 catheters, and 90,191 catheter days) comparing ALS to a standard heparin lock in CRB prevention. While four of the studies included tunnelled catheters, only one included exclusively non-tunnelled catheters, and three studies included both tunnelled and non-tunnelled catheters. ALS significantly reduced the risk of CRB (risk ratio: 0.32; 95% CI: 0.10–0.42; p<0.05). The authors concluded that the significant reduction in the incidence of CRB achieved in the ALS groups was similar to published reports from units with low bacteraemia incidence and, presumably, stricter hygienic measures. Furthermore, the limited follow up of the studies included in this meta-analysis did not allow for the assessment of the onset of adverse events or bacterial resistance with longer use of lock therapy.
In 2008, Jaffer et al.36 performed a meta-analysis of seven studies including a total of 624 patients and 819 catheters (448 tunnelled, 371 non-tunnelled) to determine the efficacy of ALS in reducing CRB in HD patients. Catheter-related infection was 7.72-fold less likely when using ALS times (95% CI: 5.11–10.33; p<0.05). The absence of mechanical complications, such as catheter occlusion, was another positive effect observed in the patients receiving ALS. The studies included in this meta-analysis used different concentrations of different substances, including gentamicin, minocycline, citrate, taurolidine, cefotaxime, and cefazolin. The major limitation of this review was the relatively short duration of follow up of the included studies, which did not allow for the opportunity to assess long-term adverse events, such as development of antibiotic resistance and systemic toxicity.
Yahav et al.37 conducted a systematic review of 16 randomised controlled trials that compared single or combination antimicrobial catheter lock solutions with heparin or another antimicrobial for the prevention of infections in HD patients. A total of 11 trials assessed antibiotic catheter lock solutions, 5 trials assessed nonantibiotic antimicrobial catheter lock solutions, and all trials compared the intervention with heparin. The rates of CRB were significantly lower with antibiotic catheter lock solutions compared with heparin lock alone, both per patient (RR: 0.44; 95% CI: 0.38–0.50; p<0.05; all 11 trials included) and per catheter day (RR: 0.37; 95% CI: 0.30–0.47; p<0.05). Catheter removal rates were significantly lower in the intervention group per patient (RR: 0.35; 95% CI: 0.23–0.55; p<0.05; 5 trials; 552 patients) and per catheter day (RR: 0.34; 95% CI: 0.21–0.55; p<0.05; 135,769 catheter days). The emergence of clinically significant resistant strains was reported in 5 trials, including 316 patients receiving intervention and 211 control patients. Only one case of gentamicin-resistant S. aureus was reported in a patient receiving gentamicin and citrate during 16 months of follow-up. ESI were reduced in the intervention group but without statistical significance. In studies of nonantibiotic antimicrobial catheter lock solutions, CRB rates were significantly lower with ALS than with heparin alone per patient (RR: 0.46; 95% CI: 0.29–0.71; p<0.05; 4 trials; 642 patients) and per catheter day (RR: 0.48; 95% CI: 0.30–0.76; p<0.05; 60,149 catheter days).
In 2011, Snaterse et al.38 performed a systematic review with the aim of summarising the evidence on the effectiveness of antibiotic-based catheter lock solutions in preventing BSI in oncology and HD patients and neonates at high CRB risk. Meta-analysis of nine trials showed a significant benefit in favour of the antibiotic-based solutions in HD patients with tunnelled catheters. CRB baseline risk was 3 per 1,000 catheter days, corresponding with a number needed to treat of three patients to prevent one CRB. The authors concluded that to determine the efficacy of the routine use of antibiotic lock solutions in HD patients, other factors should be considered, such as the side effects of antibiotics including the induction of microbial antibiotic resistance and cost-effectiveness.
In 2014, Zhao et al.39 published a meta-analysis that included 13 randomised studies with 1,770 patients and 221,064 catheter days followed up for 5 years, comparing 4% sodium citrate versus heparin (1,000 U/mL) locks. The rate of CRB was significantly lower in the citrate group (HR: 0.39; CI 95%: 0.27–0.56; p<0.001) when it was associated with other substances, such as gentamicin (p<0.001) or taurolidine (p=0.003).
Taurolidine is a taurine derivative that binds to the wall of bacteria and fungi, promoting the death of these agents. This acts as a disinfectant without inducing bacterial resistance induction.8 Previous studies have shown that taurolidine has been able to reduce CVC biofilm in vitro and in vivo.40,41 In relation to locking prophylactic therapy with taurolidine, two meta-analyses were published between 2013 and 2014. The first included six randomised controlled trials (431 patients, 86,078 day catheters), the use of taurolidine solutions in lock of CVC (HD, nutrition parenteral, and paediatric oncology patients) was significantly associated with a reduction in the incidence of CRB compared to heparin (RR: 0.34; 95% CI: 0.21–0.55; p<0.0001).42 However, only the reduction in the number of CRB by Gram-negative bacteria was statistically significant with the use of the taurolidine lock (RR: 0.27; 95% CI: 0.11–0.65; p<0.05). There were no differences between groups (taurolidine versus heparin) in relation to catheter occlusion due to thrombosis, with no bacterial resistance to taurolidine in the studies evaluated. However, the authors conclude that the results should be analysed with caution because of the small sample size of the studies and lack of methodological rigor. In 2014, Liu et al.43 also published a meta-analysis and systematic review comparing locking with taurolidine versus heparin in patients on CVC and risk of infection (HD patients, paediatric patients with onco-haematological diseases, and use of chemotherapy or parenteral nutrition) with an overall reduction in the incidence of CRB (RR: 0.47; 95% CI: 0.25–0.89; p<0.05) but with no effect on infections caused by Gram-positive bacteria. The incidence of thrombosis differed between the groups with the highest percentage of events in the taurolidine group (RR: 2.11; 95% CI: 1.16–2.09; p<0.05). Due to encompassing only three randomised trials, in addition to the heterogeneity of the study populations, protocols, and results definition, this meta-analysis has limits for interpretation.43
Few studies have found the impact of prophylactic lock therapy on CVC in the prevention of catheter ESI. In 2014, a meta-analysis was published of 23 randomised studies, 16 of which were in patients with HD, with a 69% reduction in central-line blood stream infections, defined as the presence of laboratory-confirmed CRB in any patient with CVC at the time or within 48 hours prior to infection, using antimicrobial lock solutions compared to heparin (RR: 0.31; 95% CI: 0.24–0.40; p<0.05) and with a 32% reduction in ESI (RR: 0.68; 95% CI: 0.49–0.95; p<0.05).44 A possible justification for this effect would be the extravasation that occurs in the CVC, depending on the density of the solution used, type and site of the catheter and position of the body, doses of antimicrobials close to the minimum inhibitory concentration for some pathogens, and consequent systemic maintenance of the subcutaneous tissue near the catheter orifice, reducing ESI rates. There were no differences in all-cause mortality among 13 studies that analysed this outcome (RR: 0.84; 95% CI: 0.64–1.12; p<0.05). The authors also performed sensitivity analyses to assess the effect of lock therapy on centres with low BSI rates, including studies with <1.15 events per 1,000 day catheters (6 trials) and found that the relative rate of BSI reduction remained significant in the subanalysis (RR: 0.32; 95% CI: 0.17–0.60; p<0.05).
Recently, two other meta-analyses were published with different approaches, which also observed the superiority of the substances in lock in relation to rates of catheter-related CRB.
In 2016, Wang et al.45 published a Cochrane review of 27 randomised studies with a mean follow-up of 6 months, comparing lock therapy with alternative anticoagulant solutions (19 studies with 2,216 patients), systemic anticoagulant agents (6 studies with 664 patients), and lock with low or no dose of heparin (2 studies with 123 patients), mainly with heparin 5,000 IU/mL (used in 17 studies). The primary end point was evaluation of catheter dysfunction, with no statistical difference in the three groups studied. In the individual agent analysis, recombinant tissue plasminogen activator was the only lock solution that showed reduction in catheter malfunction (RR: 0.58; 95% CI: 0.37–0.91; p<0.05).
Regarding the secondary endpoints, there was a significant reduction in CRB rates only in the group with lock of alternative anticoagulant solutions (HR: 0.46; 95% CI: 0.36–0.66; p<0.05), but it was not possible to evaluate CRB in the group with a low or no dose of heparin. In the individual analysis of alternative solutions, except ethanol, all other lock therapies reduced the incidence of CRB (citrate, antibiotics, and recombinant tissue plasminogen activator). However, the interpretation of the evidence from the study is limited by the variations in the interventions tested and the results reported, and randomised trials of high methodological quality are required.
The second study, published in 2017 by Zang et al.,46 was a meta-analysis that, unlike the others cited, compared the effectiveness of antimicrobial solutions in locking each other in the prophylaxis of catheter-related infections in HD. This Bayesian Network meta-analysis included 18 studies with 2,395 patients and analysed 10 lock therapy strategies (including the control group). Gentamicin + citrate (overall response [OR]: 0.07; 95% CI: 0.00–0.48; p<0.05) and gentamicin + heparin (OR: 0.04; 95% CI: 0.00–0.23; p<0.05) were significantly more effective in reducing rates of catheter-related CRB when compared to the use of heparin-only locks. Regarding the incidence of ESI and all-cause mortality, no significant difference in the intervention effect was detected for all lock solutions when compared to heparin. All solutions were similar for catheter-related CRB, ESI, and all-cause mortality, when compared. This meta-analysis is important to compare the effect of several solutions in locking each other, besides making an analysis of probability of effect among them; however, due to the high heterogeneity between the studies, a small number of trials evaluated for some of the interventions and methodological quality of the work, the results should be interpreted with caution.
Table 1 summarises the major characteristics of the meta-analysis studies from the last 10 years on prophylactic antibiotic topical and lock therapy for HD-tunnelled catheters.
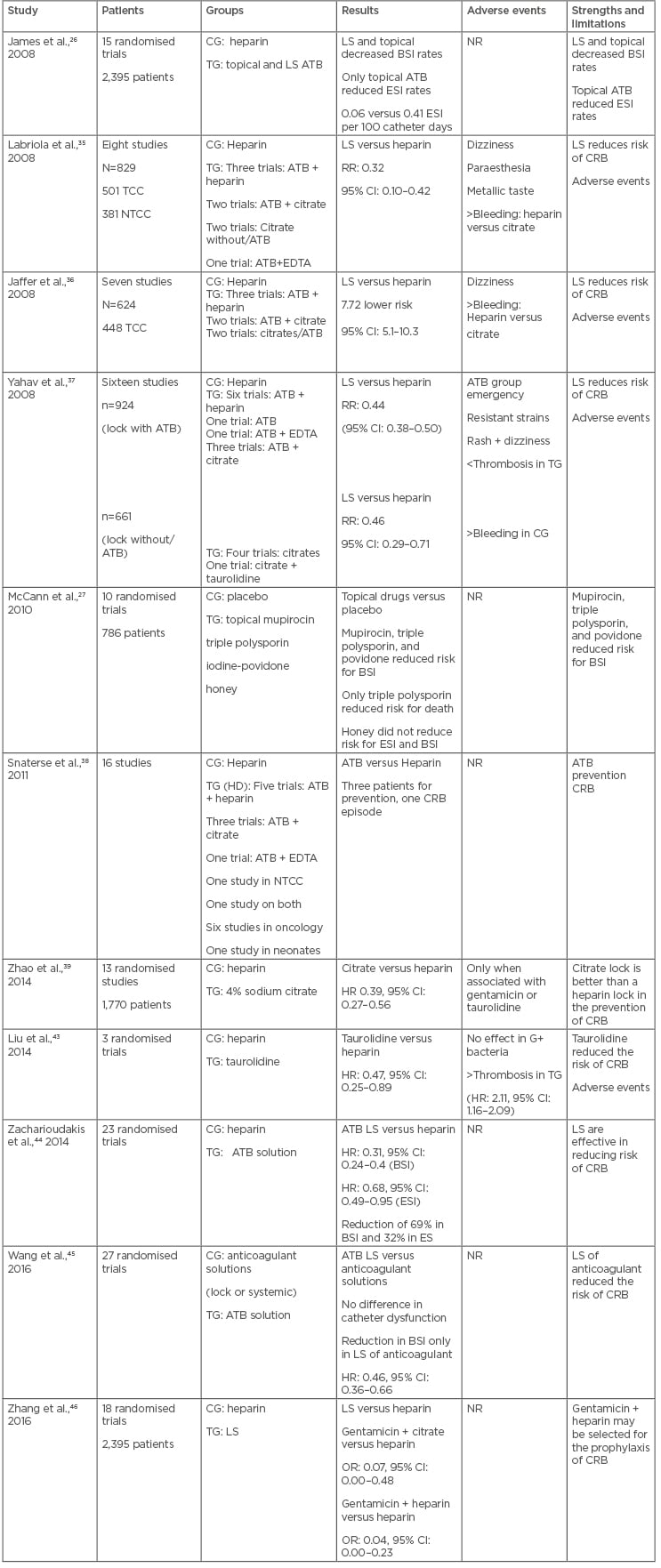
Table 1: Summary of the meta-analyses using prophylactic antimicrobial therapy in haemodialysis central venous catheters.
ATB: antibiotic; BSI: bloodstream infection; CD: catheter day; CG: control group; CRB: catheter-related bacteraemia; CVC: central venous catheter; DS: dialysis session; EDTA: ethylenediaminetetraacetic acid; ESI: exit site infection; LS: lock solution therapy; MuRSA: mupirocin-resistant Staphylococcus aureus; NR: not reported; NTCC: non-tunnelled central venous catheter; TCC: tunnelled central venous catheter; TG: treatment group; tPA: tissue plasminogen activator.
CONCLUSION
Although recent meta-analyses have shown favourable results related to the use of antimicrobial lock therapy in reducing the rates of catheter-related infection, the 2006 NKF KDOQI guidelines still do not recommend ALS or topical antibiotic for the prophylaxis of CRB in patients on HD.7 According to the 2011 CDC guidelines, their use should be reserved to patients with a long-term catheter, who have a history of multiple CRB, despite optimal maximal adherence to aseptic techniques.
As final recommendations based on several studies discussed, topical antibiotics and catheter lock solutions are the primary means of preventing BSI and should be used, but the risk of emergence of organisms resistant to the antibiotics used should be considered. Further studies to assess the impact of long-term use of intraluminal and topical antimicrobial on the development of bacterial resistance are warranted.








