Abstract
In an ideal world, every condition would have a sensitive and specific marker that could be measured in a noninvasive or minimally invasive way. Instead, the medical community depends on invasive biomarkers, which carry inherent risks, to make a diagnosis and plan treatment. In this review article, the current state of research into biomarkers for a range of kidney diseases is discussed, beginning with those biomarkers that are already in clinical use and then moving to conditions for which no validated biomarker yet exists. This review focusses on diabetic nephropathy at the proteinuric end of the spectrum and antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis at the nephritic end. An interesting feature is that the same biomarker, monocyte chemoattractant protein-1 (MCP-1, also known as CCL2), has been identified as a potential target in both conditions, which suggests a shared pathogenic process that results in two very distinct clinical presentations. One of the major limiting features of research into this area, particularly for ANCA-associated vasculitis, is the recruitment of a sufficient number of patients to generate strong enough evidence to justify the biomarker’s routine use; this overlap in biomarkers may enable research in one condition to be applied more generally. In addition to their role as biomarkers, these molecules are also therapeutic targets, and some early research has been carried out to investigate this. Overall, this review brings together research from diverse fields to focus attention on the outstanding areas and the future areas that warrant further investigation.
INTRODUCTION
The term ‘biomarker’ is commonly used, but a precise definition has not been agreed upon. One definition has been provided by the World Health Organization (WHO), which has defined biomarkers as ‘any substance, structure, or process that can be measured in the body or its products and influence or predict the incidence of outcome or disease.’1 In this review, the authors will focus more specifically on antibodies and cytokines that can be used as surrogate markers for the presence of renal diseases, assessing disease severity and monitoring progression.
There are a number of reasons why biomarkers are useful (Table 1). A major aim of the use of biomarkers is to prevent progression to end-stage renal failure and reduce the burden of organ replacement therapy. However, biopsy still has a crucial role to play in cases of diagnostic uncertainty or to assess the severity of a presentation. The use of noninvasive biomarkers in a way that complements, or avoids the need for, biopsy in certain cases, is a topic of much research.

Table 1: The advantages of using biomarkers in the diagnostic and treatment settings.
In Goodpasture’s syndrome, biomarkers have been clearly demonstrated to be useful. In the syndrome, a specific antibody to the glomerular basement membrane (anti-GBM) leads to rapidly progressive glomerulonephritis; this antibody can be detected in the serum, traditionally using enzyme immunoassays and immunofluorescence of renal biopsy specimens. While estimates vary, a recent study found a specificity and sensitivity of serum anti-GBM antibodies for Goodpasture’s syndrome of 85.4% and 41.2%, respectively.2 Therefore, detection of anti-GBM antibodies strongly supports the diagnosis; however, approximately 10% of patients do not have identifiable circulating antibodies, and thus renal biopsy is still recommended.3 There is some evidence suggesting that higher titres of anti-GBM antibodies are associated with worse renal survival4,5 and stronger evidence that antibodies against specific conformational epitopes may correlate with worse prognosis.6 A fall in anti-GBM antibody titres usually correlates with clinical improvement, and in most cases recurrence does not occur. However, there is evidence from case reports that rising titres may associate with or precede clinical relapses.7
The archetypal example of the use of biomarkers in nephrology comes from the diagnosis of systemic lupus erythematosus (SLE). Antinuclear antibodies are the most sensitive biomarker for SLE, but they are the least specific, as 1 in 3 healthy individuals have detectable antinuclear antibodies, albeit at relatively low titres.8 Anti-double stranded DNA (anti-dsDNA) antibodies are more specific for SLE, but there is a significant loss of sensitivity as assays may be negative early in the disease course. However, anti-dsDNA can be useful in disease monitoring because antibody levels typically increase prior to an exacerbation.9 When anti-dsDNA levels are negative, the anti-extractable nuclear antigens become more useful, but they are not very specific, and there is little evidence that anti-extractable nuclear antigen titres reflect disease activity.10 Reduction in complement C3 and C4 levels are also useful in monitoring of SLE, but play little role in diagnostics. These markers are all routinely used, though their sensitivities are relatively low; estimates vary, but quoted values are shown in Table 2.11 William Egner produced an excellent table looking at when various markers are clinically useful.12 There does not appear to have been research carried out into the sensitivity and specificities achieved through various combinations of these biomarkers that may improve the values. More recently, interest has been generated by research into the use of urinary proteomics in patients with lupus nephritis. Surface enhanced laser desorption/ionisation time-of-flight MS (SELDI-TOF MS) techniques have identified proteins in the urine that distinguish between active and inactive lupus nephritis.11 While these have not been validated for the initial diagnosis, they provide a potential mechanism for early diagnosis of relapse. However, the technique has not become routine in current clinical practice.
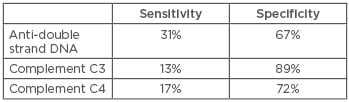
Table 2: Sensitivity and specificity of biomarkers in lupus nephritis.11
During undergraduate medical education, renal diseases are often taught as being on a spectrum from pure proteinuria and nephrotic syndrome at one extreme to haematuria and nephritic syndrome on the other. To look at the current research into the translation of clinical markers from the lab to the clinic, this review focusses on two conditions at near opposite ends of the spectrum: diabetic nephropathy and antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV). By selecting these two conditions, the authors hope to highlight both the differences and surprising similarities in the use of biomarkers in these conditions.
DIABETIC NEPHROPATHY
Diabetic nephropathy may seem an odd choice; it is a secondary cause of nephropathy traditionally thought to be due to the deposition of extracellular matrix resulting in fibrosis. However, both experimental models and renal biopsies have shown an increased number of macrophages compared with controls.13 A number of groups have identified signalling molecules that mediate these inflammatory markers. The best characterised are transforming growth factor-beta (TGF-β), connective tissue growth factor (CTGF), and monocyte chemoattractant protein-1 (MCP-1).
Transforming Growth Factor-Beta
TGF-β is a cytokine that is essential for normal organ development and function, yet it is associated with pathological fibrosis characterised by excessive extracellular matrix accumulation.14 TGF-β expression is increased in the renal biopsies of patients with diabetic nephropathy mirroring its important role as a mediator in experimental models.15 TGF-β production is stimulated at the transcriptional level by high glucose concentrations,16 and expression levels are closely correlated with the degree of glycaemic control.17 Increased TGF-β causes downstream profibrotic pathways, including synthesis of CTGF, resulting in progressive tubulointerstitial fibrosis and tubular atrophy.18
There have been no large studies looking at whether serum or urinary TGF-β levels are associated with the risk of diabetic nephropathy. However, a recent meta-analysis found that TGF-β1 levels were significantly increased in patients with diabetic nephropathy compared to those with diabetes alone and healthy controls.19 This is supported by studies that have found that a renin-angiotensin system blockade leads to a reduction in urinary TGF-β1 levels in diabetic nephropathy,20 an effect shown to be enhanced by vitamin D replacement.21 Overall, the evidence for the use of TGF-β as a biomarker is not available yet, but a potential problem arises from its lack of specificity to diabetic nephropathy.
Connective Tissue Growth Factor
CTGF is produced by mesangial and tubular epithelial cells and is present in the glomerulus of patients with diabetes. In mouse models, the amount of CTGF immunostaining correlates with the duration of diabetes.22 There is good evidence that CTGF is a crucial mediator of TGF-β-stimulated matrix protein expression, which leads to scarring and inflammation. Specifically, CTGF mediates TGF-β-induced increases in fibronectin and collagen type I.23,24 It has been demonstrated that there are increased amounts of CTGF in urine samples of patients with diabetic nephropathy25 and that elevated levels of CTGF are predictive of an increase in microalbuminuria in the next year.26
Urinary CTGF has been demonstrated to correlate clinically with degree of albuminuria in patients with Type 1 diabetes mellitus. In the subgroup of patients with macroalbuminuria, urinary CTGF was lower in patients receiving angiotensin converting enzyme (ACE) inhibitor treatment than those not yet receiving ACE inhibitor treatment.27 Plasma CTGF has been shown to be an independent predictor of end-stage renal failure and mortality in Type I diabetic nephropathy.28 A Phase I trial of an anti-CTGF monoclonal antibody in patients with diabetes showed a reduction in microalbuminuria, although the trial was not designed to assess efficacy of treatment.29 There is therefore good evidence for a role for CTGF as a marker of diabetic nephropathy and a potential treatment target.
However, while much of the focus has been on CTGF in diabetic nephropathy, there is evidence supporting its role in other conditions. CTGF expression has been shown to be increased in a range of inflammatory, glomerular, and tubulointerstitial lesions associated with cellular proliferation and matrix accumulation,30 which included IgA nephropathy, chronic transplant rejection, crescentic glomerulonephritis, lupus nephritis, and membranoproliferative glomerulonephritis. In patients with non-diabetic chronic kidney disease, treatment with angiotensin receptor blockade leads to reduction of urinary CTGF levels, although plasma CTGF levels are unaffected.31 Therefore, while the presence of CTGF in the urine may suggest a pathogenic process, it does not appear to be sufficiently specific as a diagnostic marker for diabetic nephropathy.
Monocyte Chemoattractant Protein-1
MCP-1 is a potent chemokine that activates monocytes and macrophages.32 There is good evidence for its role in diabetic nephropathy. It has been demonstrated that renal mesangial cells produce MCP-1 in response to high concentrations of glucose.33 MCP-1 knockout mice are protected from streptozotocin-induced diabetic nephropathy.34 There is controversial evidence over the role of a genetic polymorphism in the MCP-1 gene leading to a higher frequency of diabetes in some studies,35 or an increased risk of diabetic nephropathy in diabetic patients but not an overall increase in diabetes, as shown in other studies.36
MCP-1 is detectable in urine from patients with diabetic nephropathy.37 Increased levels of MCP-1 correlate with the decrease in estimated glomerular filtration rate in diabetic patients over 6 years of follow-up, and this is an independent predictor from baseline proteinuria.26 In patients with macroalbuminaemia, urinary MCP-1 is an independent risk factor for chronic kidney disease progression.38 Intensive insulin therapy has been shown to lower urinary MCP-1 levels in Type 2 diabetics with microalbuminaemia,39 but this was only over a short time period and did not correlate with any clinical improvement.
The most recent data comes from groups looking to validate the use of biomarkers in diabetic nephropathy. Verhave et al.40 analysed seven urinary biomarkers in 83 patients followed over 2 years. They identified MCP-1 along with TGF-β as independent predictors of a fall in kidney function. The larger study from Nadkarni et al.41 used samples from 380 patients enrolled in the ACCORD trial and found only MCP-1 as a predictor of sustained decline in renal function over 5 years of follow-up, independent of baseline kidney function or degree of proteinuria. MCP-1, therefore, appears to provide some prognostic information in diabetic nephropathy. However, it is not specific to diabetic nephropathy, with some evidence for its relevance in lupus nephritis42,43 and its potential use in AAV discussed below.
One additional angle to the MCP-1 story comes from a recent Phase II clinical trial studying the use of MCP-1 receptor inhibitors in the treatment of diabetic nephropathy.44 Patients were recruited to receive either placebo or one of two doses of inhibitor for 1 year and the results demonstrated a significant reduction of albuminuria in both treatment arms. Therefore, MCP-1 appears to be not only a marker of diabetic nephropathy but also a potential therapeutic target.
ANTINEUTROPHIL CYTOPLASMIC ANTIBODY-ASSOCIATED VASCULITIS
AAV are a group of conditions associated with the presence of ANCA, either perinuclear ANCA, targeted against myeloperoxidase, or cytoplasmic-ANCA, directed against proteinase 3. However, while these markers are sensitive and specific for diagnosis of an AAV, at 85.5% and 98.6%, respectively, for combined ANCA testing, the value of serial monitoring is less clear.45 A meta-analysis has shown there is a modest association between relapse and a rise or persistence of ANCA during remission,46 with some studies finding a stronger effect if patients without renal involvement were excluded.47 Due to these limitations, there has been a broader search for alternative biomarkers. Some biomarkers that have been identified as potentially of interest include serum calprotectin, serum anti-pentraxin 3 antibodies, and urinary soluble CD163 (sCD163).48 Of these, sCD163 shows the most promise; however, it is more useful for serial monitoring of disease activity in patients with an established diagnosis because it is not sufficiently specific for the initial diagnosis.49 The best overall marker that has been identified so far is MCP-1.50,51
MCP-1 was first highlighted as a potential biomarker by Tam et al.,50 who showed that urinary MCP-1 was significantly higher in patients with active renal vasculitis compared to those with inactive vasculitis, patients with active extra-renal vasculitis, healthy volunteers, or controls between whom there were no significant differences. The difference was not seen in serum MCP-1 levels. This study was supported by work from Lieberthal et al.52 who also identified MCP-1 as the best discriminator for active and inactive vasculitis but found it nonspecific for renal flares. Both studies looked at MCP-1 as a diagnostic tool and were followed by a study by Ohlsson et al.,53 which demonstrated that it is also a marker of poor prognosis. A recent systematic review looked at 161 marker molecules, and of these MCP-1 had the highest sensitivity (100%) and a reasonable specificity (75%).51 The only other markers that achieved statistical significance were a C-reactive protein cut-off of 21.6, C3a in serum, and C5a in urine.51
The major limitation in the identification of biomarkers for AAV is the sample sizes. In the systematic review, the sample sizes ranged from 16–22 patients with active and inactive vasculitis.51 Even large renal centres struggle to recruit sufficient numbers to detect small differences. More recently, interest has focussed on the cytokines APRIL (A proliferation-inducing ligand) and BAFF (B cell activating factor of the TNF family). There is some evidence that these associate with disease activity,54,55 but there is currently insufficient data to draw conclusions.
DISCUSSION
The field of biomarkers is subject to extensive research, but very few markers have successfully transitioned from scientific interest to clinical usage. SLE provides the benchmark for the use of biomarkers in clinical practice, with a range of autoantibodies providing clinically useful information. However, there have been minimal further advances since the discovery of these autoantibodies, despite large volumes of work into the cytokines associated with renal diseases. Table 3 compares the biomarkers that have been discussed in this article.
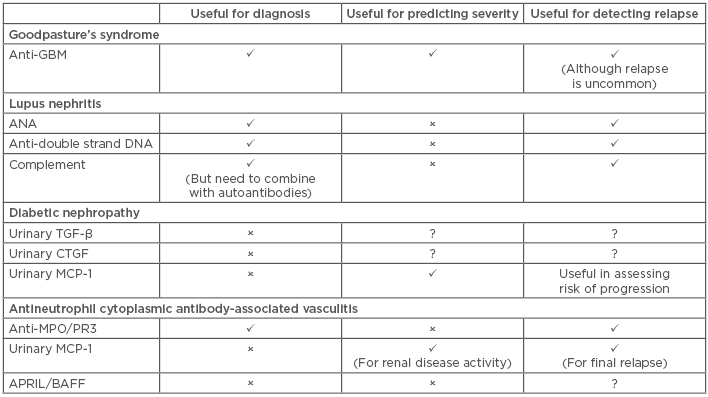
Table 3: Comparison of the use of key biomarkers in Goodpasture’s syndrome, lupus nephritis, diabetic nephropathy, and antineutrophil cytoplasmic antibody-associated vasculitis.
✓: yes; ✕: no; ?: unknown or uncertain; APRIL: a proliferation-inducing ligand; ANA: antinuclear antibodies; BAFF: B cell activating factor of the TNF family; CTFG: connective tissue growth factor; GBM: glomerular basement membrane; MCP-1: monocyte chemoattractant protein-1; MPO: myeloperoxidase; PR3: proteinase 3; TGF-β: transforming growth factor-beta.
Most of the biomarkers that have been discussed in this article have been identified using classical biomarker ELISA assays. The field is not only expanding in terms of the number of biomarkers identified but also the techniques used to identify them. Recently, urinary proteomics has been used to identify neprilysin and VCAM-1 as potential biomarkers for diabetic nephropathy,56 although further studies are needed to validate these findings. There is also increasing interest in single-cell transcriptomics, which is a technique for the analysis of gene expression at the level of the individual cell and has been used to identify 21 distinct cell types within the kidney.57 A deeper mechanistic understanding of diseases may help in identifying combinations of biomarkers that together provide the specificity which individual biomarkers currently lack.
One of the major challenges is the lack of large-scale trials in this area. The number of patients who present with AAV, and the acuteness of these presentations, make recruitment into prospective clinical studies more challenging, thus limiting the strength of the evidence obtained. Stronger data are seen in the diabetic cohorts who typically develop renal impairment more slowly and progressively, and in much larger numbers. Can the research community generate enough confidence in the data generated so far that will allow for their use in clinical trials? Or even application for individual patients?
An interesting finding is that urinary cytokines, particularly MCP-1, appear to be an important marker at both ends of the spectrum of renal diseases. While this limits their specificities, it may inform researchers of the general mechanism of kidney injury not unique to an individual condition. Therefore, can it be inferred that such cytokines are markers of general inflammation and generalisable across a wide range of conditions? This may mitigate some of the challenges in AAV research by making available a wider cohort of patients, with a range of renal diseases.
To date, no biomarker has been identified that has the sensitivity, specificity, and prognostic value to replace a renal biopsy, so the biopsy remains the gold standard for diagnosis. The biomarkers may prove to be more useful not for initial diagnosis but in monitoring disease progression when the underlying disease is already known and therefore the lack of specificity is less problematic. However, biomarkers have not made the leap into the clinic, as currently the evidence is not sufficiently robust. Future research focussed more generally on their utility in renal disease rather than any specific condition may provide sufficient data to support their use.
The biomarkers discussed are not only increased in multiple kidney diseases (of both immune and nonimmune origins) but also in disorders of other organs; therefore, it is not surprising that urinary biomarkers currently show more promise in kidney disease as they localise the inflammation to the urogenital tract. Instead of attempting to replace the renal biopsy, the significance of these markers may be strengthened by the correlation of their concentration with the histopathological findings, an area which has not been fully investigated.
The biomarkers discussed have primarily been investigated for their use in identifying those with or without a condition. Another area that has been less well studied is their use in identifying individuals who will or will not respond to a particular treatment. This would potentially enhance the probability of therapeutic success.
However, these biomarkers may be mechanistic, providing prognostic information because they are directly involved in the renal damage themselves. The levels can be measured and clinical information inferred. Alternatively, the biomarkers could be important potential therapeutic targets; clinical trials are ongoing that are examining anti-CTGF antibody and MCP-1 receptor antagonists, and such therapies may indeed be the legacy of decades of research into these biomarkers.
CONCLUSION
This article reviewed the current state of biomarker knowledge for two important renal conditions. The role of urinary MCP-1 as a biomarker for diabetic nephropathy and AAV, as well as the potential of MCP-1 to become a therapeutic target, has been highlighted. However, MCP-1, along with other biomarkers, lacks the specificity for a particular disease and instead reflects a state of inflammation and fibrosis.
The field of biomarker research has huge potential that has not yet been fully realised. The authors have highlighted the small number of patients that can be enrolled into clinical trials as a key obstacle to the progression of the research into this field. However, the lack of specificity of these markers may allow us to assess their use across a group of conditions, thereby increasing the number of patients. Finally, avenues for future research, namely new techniques to identify biomarkers, using biomarkers in conjunction with histopathology rather than attempting to replace it, using biomarkers to identify likely responders, and biomarkers as potential therapeutic targets, have been discussed.








