Abstract
Introduction: Chronic kidney disease (CKD) affects the pituitary-thyroid axis and peripheral metabolism of thyroid hormones, thereby causing dysfunction of thyroid hormones. This study aimed to highlight the correlation between thyroid dysfunction and the staging of chronic kidney disease.
Methods: Fifty patients with CKD were studied between 2014–2017 in a tertiary care centre in Western India. These patients were split into a subgroup for Stage 4 and 5 CKD, based upon estimated glomerular filtration rate as per the Kidney Disease Improving Global Outcomes (KDIGO) guidelines. Thyroid function tests and lipid levels were compared in the different CKD subgroups by analysis of variance. Pearson’s correlation coefficient was used to evaluate the thyroid dysfunction with respect to degree of renal dysfunction in the study population.
Results: Thyroid abnormalities were seen in 68% of the author’s patients. Euthyroid sick syndrome (ESS) was the most prevalent thyroid hormonal abnormality seen in 32% (n=16), followed by hypothyroidism category seen in the remaining 36% (n=18) of the study population. Of these, 18% (n=9) of the patients had subclinical hypothyroidism, while the remaining 18% (n=9) manifested overt hypothyroidism. Results showed that ESS had a positive correlation with estimated glomerular filtration rate (r=0.822; p<0.001). No significant differences were found between groups in thyroxine or thyroid-stimulating hormone (p>0.05). Linear regression in unadjusted analysis revealed that deranged low-density lipoprotein levels was found to be significantly associated negatively with hypothyroidism (p<0.001) in patients with CKD.
Conclusion: Patients with Stage 4 and 5 CKD have many hormonal disturbances, of which ESS is a common occurrence, and has a significant association with dyslipidaemia, increasing morbidity in these patients. Hypothyroidism is more prevalent in patients with severe renal dysfunction, as a result of higher uremic milieu in these patients.
Key Points
1. The association between chronic kidney disease (CKD) and thyroid has been well studied, but its impact in patients with Stage 4 and Stage 5 CKD remains an unsolved problem.
2. In this study, the author demonstrated the correlation of tri-iodothyronine, thyroxine, and thyroid-stimulating hormone with metabolic parameters of CKD.
3. The study showed that low tri-iodothyronine and low thyroxine syndrome progressively increased as the severity of chronic kidney disease increased, which may impact progressive worsening of estimated glomerular filtration rate.
INTRODUCTION
A spectrum of various pathophysiological processes associated with a progressive worsening in estimated glomerular filtration rate (eGFR) and abnormal kidney function are encompassed in chronic kidney disease (CKD).1,2 Despite various aetiologies, irreversible destruction of nephrons ultimately culminates into CKD, resulting in alteration of the internal milieu that affects each and every system in the body, including the thyroid hormonal system. In the clinical scenario of patients with CKD, thyroid gland disorders such as hypothyroidism and euthyroid sick syndrome (ESS) occur very often, especially in Stage 5 CKD.3-5 However, the directionality of the association and mechanistic link between kidney disease and ESS remain widely unknown. In patients with CKD, a pivotal role is played by the inflammatory cytokines and oxidative stress in the pathogenesis of ESS. A study by Lo et al.3 indicated the increasing prevalence of subclinical primary hypothyroidism from 5.4% to more than 20.0% when the eGFR reduced from >90 mL/min/1.73 m2 to <60 mL/min/1.73 m2. In addition to the above, Song et al.4 indicated the morbidity of low tri-iodothyronine (T3) syndrome to be increased in patients with CKD. In patients with hypothyroidism, clinically and statistically important reductions in eGFR are seen, which could be attenuated by using adequate thyroid hormone replacement therapy.6,7 Studies also demonstrated the clinical and subclinical hypothyroidism states to be independent risk factors for cardiovascular death and all-cause mortality, which could be the effects of worsening atherosclerosis in coronary and peripheral vessels and hyperlipidaemia.8-10 Due to the dearth of available data to prove the relationships between thyroid hormones and eGFR, gender, age, and various other biomarkers such as haemoglobin, electrolytes, and lipid profile in patients with Stage 4 and 5 CKD, the author performed such kinds of analysis to explore the relationships between these biomarkers in patients with Stage 4 and 5 CKD and thyroid hormones.
MATERIALS AND METHODS
Study Design
A prospective, cross-sectional study was conducted between 2014–2016 at a tertiary healthcare centre, in patients who were admitted and registered into the system. After approval by the Institutional Review Board, the study was performed in accordance with the Declaration of Helsinki. An informed consent was sort and obtained from all participants on admission to the hospital, and prior to the required investigations.
Study Population
A total of 50 patients hospitalised with CKD, and not on dialysis, were recruited. These randomly chosen participants had Stage 4 and 5 CKD (i.e., GFR <30 ml/min), and were patients who fulfilled the criteria for CKD (uraemic symptoms for ≥3 months, with decreased eGFR and ultrasound evidence of chronic kidney disease), and who were on conservative management. The author excluded patients younger than 18 years of age; females who were pregnant; patients with acute kidney injury, or undergoing renal replacement therapy; patients who were receiving concurrent treatment with drugs that could affect thyroid hormones (amiodarone, lithium, methimazole, iodine, oestrogen pills, or phenytoin); or with known thyroid illness in the past. Outpatients were also excluded to prevent acute kidney injury (acute illness) causing confounding results on thyroid panels, and to prevent dropouts during follow-up.
Laboratory Measure
The electrochemiluminescence assay (Siemens, Munich, Germany) was used to test thyroid function. The normal reference range of the author’s institution was T3 (60–200 ng/dL), thyroxine (T4; 4.5–12.5 mcg/dL), ‘free’ T4 (FT4; 0.8–2 ng/dL), and thyroid-stimulating hormone (TSH; 0.3–5.5 mIU/L). Clinical hypothyroidism was defined as a condition with increased TSH, along with decreased FT4 and decreased or normal T3. Clinical hyperthyroidism was defined as a condition with a lowered TSH, along with a raised FT4, T4 and/or T3. Subclinical hypothyroidism was defined as a condition with raised TSH along with a normal FT4, T4, and/or T3. Subclinical hyperthyroidism was defined as a condition with a lowered TSH, along with a normal FT4, T4, and T3. ESS was defined as a condition with normal or a low TSH, along with low T3 and/or a low FT4 and TT4. Serum sodium, potassium, calcium, urea, and creatinine were measured by an enzymatic method called the isotope dilution mass spectrometry.11 Haemoglobin was detected by an automated haematology system. Serum lipid profile analysis (triglycerides, low-density lipoprotein [LDL] and high-density lipoprotein) using an enzymatic colorimetric method. Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) method was utilised to calculate the eGFR.12,13
Statistical Analysis
Analysis was conducted using the PASW 18.0 software (SPSS Inc., Chicago, Illinois, USA). The following was the expression of eGFR equation in software: eGFR=141 x min (Scr/ĸ,1)α x max (Scr/ ĸ,1)-1.209 x 0.993age x 1.018 (if female). ĸ was 0.9 for males and 0.7 for females; α was -0.411 for males and -0.329 for females; max indicates the maximum of Scr/K or 1, and min indicates the minimum of Scr/ĸ or 1. CKD Stage 4 and 5 definitions were in accordance with KIDGO guidelines (Stage 4: eGFR <30 mL/min/1.73 m2–≤15 mL/min/1.73 m2; Stage 5: eGFR <15 mL/min/1.73 m2). Data were expressed as mean±standard deviation. Different thyroid diseases prevalence were calculated by χ2 test. One-way analysis of variance was used to compare the levels of various biochemical markers in different CKD stages and thyroid hormones. Association between categorical factors and groups were tested using the χ2 test. For any statistical significance, a p-value of ≤0.05 was fixed. Spearman correlation analysis was used to analyse the correlations between various kidney biomarkers and thyroid hormones. Multivariate logistic regression was used to assess the adjusted effect of the factors on incidence of hypothyroidism. Factors that are statistically significant at 10% level of significance from univariate analysis and clinically significant were included in the model.
RESULTS
Patient Characteristics in Various Chronic Kidney Disease Groups
Characteristics of 50 patients recruited are depicted in Table 1. The mean age of the patients was 49.5 years, with an equal distribution of all patients above and below 50 years of age (p=0.199), and 80% of the total patients were males. The mean age of the patients in both Stage 4 and Stage 5 CKD groups did not show any statistical difference (p=0.199), with a similar distribution of patients of age above and below 50 years in Stage 4 and 5 groups (p=0.239). Similarly, the gender distribution of the patients in the Stage 4 and 5 groups were not statistically different (p=0.440). There were significant differences among the two groups in eGFR, urea, creatinine, T3, haemoglobin, sodium, potassium, and calcium (p<0.001). Patients in the Stage 5 group had a lower T3, haemoglobin, sodium, calcium, and a high potassium (p<0.001). No significant differences were found in TSH, T4, FT4, and LDL (p>0.05).
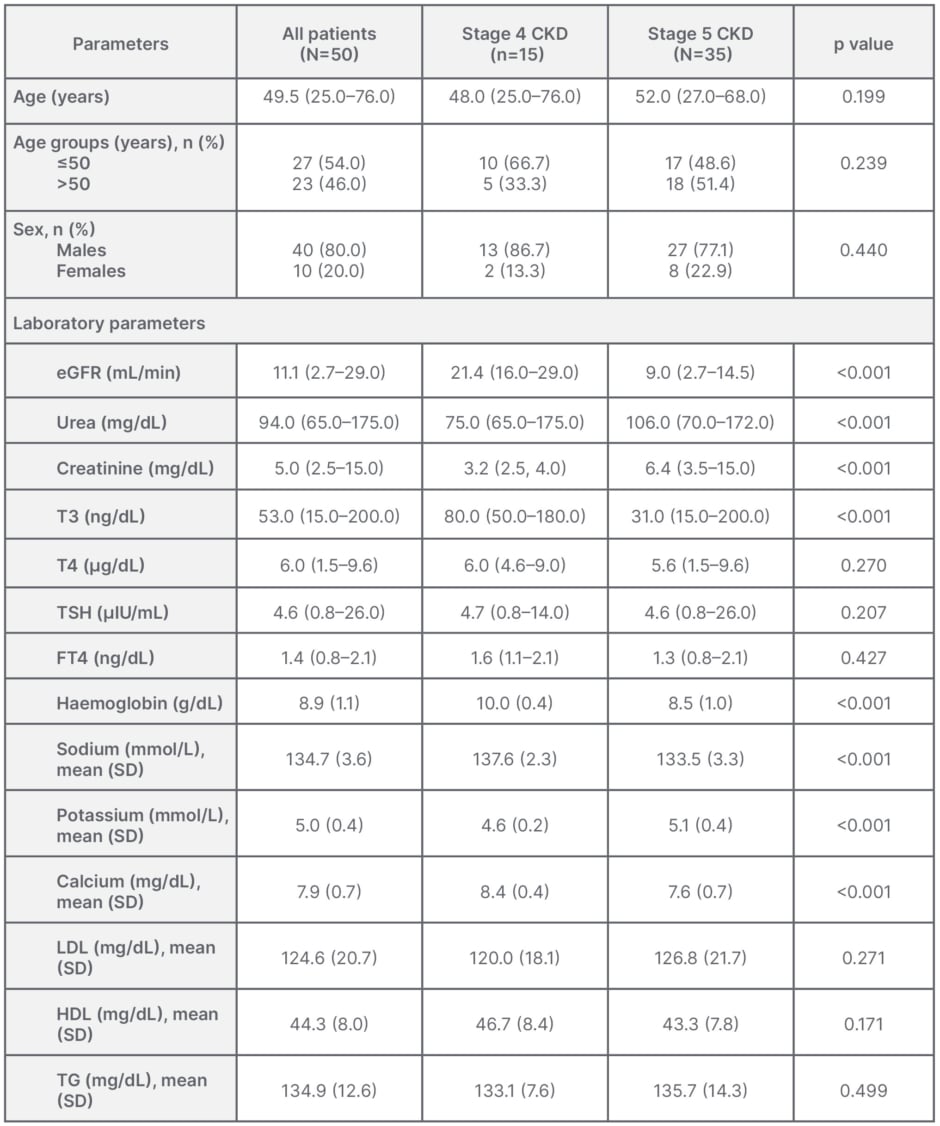
Table 1: Characteristics of patients with chronic kidney disease in different groups.
Data shown as median (range), unless otherwise specified.
CKD: chronic kidney disease; eGFR: estimated glomerular filtration rate; FT4: free thyroxine; HDL:
high-density lipoprotein; LDL: low-density lipoprotein; SD: standard deviation; T3: triiodothyronine;
T4: thyroxine; TG: triglyceride; TSH: thyroid-stimulating hormone.
Prevalence of Different Types of Thyroidism and Dyslipidaemia in Chronic Kidney Disease Groups
Subclinical and overt hyperthyroidism were not seen in either CKD group. However, there was a high prevalence of euthyroid state (normal: 32%) and ESS (32%), as compared with subclinical (18%) and overt hypothyroidism (18%) in CKD groups. The euthyroid state was more prevalent in the Stage 5 group, but this was not statistically significant (p>0.05), while ESS had a similar prevalence in both groups. The morbidity of overt hypothyroidism was 18%, and was especially high in patients with Stage 5 CKD (p<0.026). There was a high prevalence of patients with undesirable high LDL cholesterol, high triglycerides, and low HDL cholesterol levels in these groups, with no significant differences between either (p=0.948, p=0.810, and p=0.087, respectively; Table 2).
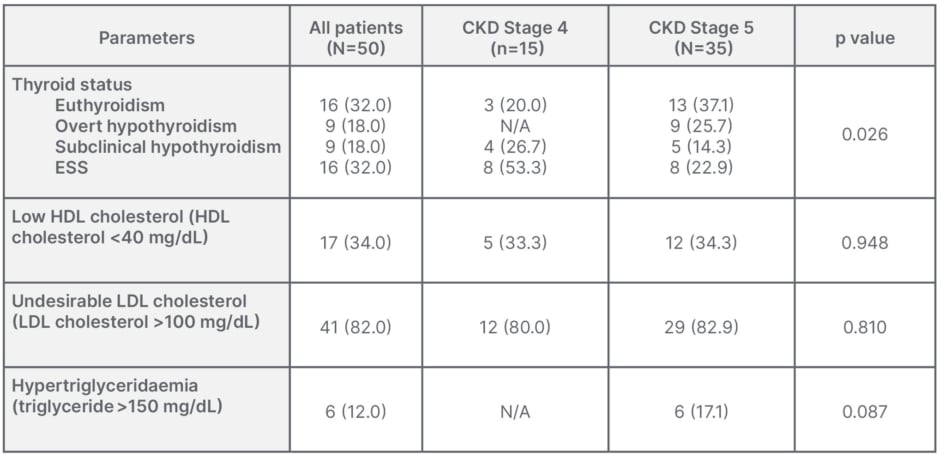
Table 2: Thyroid dysfunction and dyslipidaemia in different stages of chronic kidney disease.
Data shown as n (%).
ESS: euthyroid sick syndrome; HDL: high-density lipoprotein; LDL: low-density lipoprotein.
Correlation Analysis of Thyroid Hormone and Chronic Kidney Disease
The analysis between thyroid hormone and urea, creatinine, and eGFR were shown in Table 3. With urea and thyroid hormones, Pearson’s correlation coefficient (R) was -0.658 between T3 and urea, which had a significant statistic negative correlation (p<0.001). R was -0.302 between T4 and urea, and 0.211 between TSH and urea, both of which were statistically non-significant. With creatinine and thyroid hormones, R was -0.792 between T3 and creatinine, which had a significant statistic negative correlation (p<0.001). R was -0.305 between T4 and creatinine, and 0.231 between TSH and creatinine, both of which were statistically non-significant. With eGFR and thyroid hormones, R was 0.822 between T3 and eGFR, which had a significant statistic positive correlation (p<0.001). R was 0.309 between T4 and eGFR and -0.263 between TSH and eGFR, both of which were statistically non-significant.
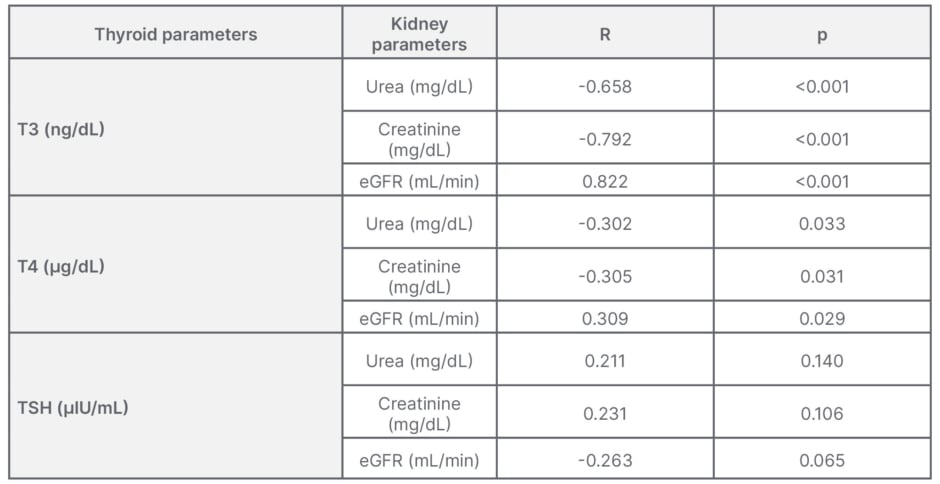
Table 3: Correlation between thyroid hormones and parameters of renal function.
eGFR: estimated glomerular filtration rate; R: correlation coefficient; T3: tri-iodothyronine;
T4: thyroxine; TSH: thyroid-stimulating hormone.
Analysis of Other Covariates with Chronic Kidney Disease Staging and Hypothyroidism
Association analysis were also conducted between the biomarkers and CKD staging. None of the covariates showed any significant association with CKD staging. Lower levels of haemoglobin showed association with CKD staging in unadjusted models, but it was not statistically significant (odds ratio [OR]: -0.334; 95% confidence interval: -0.382–0.015; p=0.073). None of the other kidney biomarkers, which included urea, creatinine, and other electrolytes showed any association with CKD staging in unadjusted models (p>0.05). Similarly, no association was seen between lipid profile and CKD staging in unadjusted models (p>0.05). LDL cholesterol demonstrated a significant negative correlation in unadjusted analysis with hypothyroidism (OR: -0.759; p<0.001), but after adjustment with other kidney biomarkers no significant correlation was found between LDL and hypothyroidism in patients with CKD (OR: 1.002; 95% confidence interval: 0.998–1.007; p=0.296).
DISCUSSION
Of the 50 patients recruited, patients were predominately males (80%), which reflects the gender discrepancy in healthcare seeking-behaviour in Western India. The analysis revealed that T3 levels change in accordance with urea, creatinine, and eGFR. Urea and creatinine negatively correlated with T3, while eGFR positively correlated with T3 levels. The most common thyroid disorder in Stage 4 and Stage 5 CKD groups is ESS. In this analysis, it was found that the frequency of ESS demonstrated a rising trend in patients with Stage 4 and 5 CKD, the number being around 32% with equal representation in both CKD groups. Stage 5 patients had an increasing trend in frequency of overt hypothyroidism of around 26%. Additionally, overt hypothyroidism had correlations with LDL. However, adjustment and multivariate analysis revealed overt hypothyroidism to be independent of LDL.
Multiple reports have shown that thyroid glands and kidneys have a close relationship,11,14,15 and that thyroid hormones play an important role in the metabolism and development of the kidneys.12 Thyroid gland dysfunction induces disorders of electrolytes, water, and lipids, amongst others. Den Hollander et al.13 demonstrated the kidneys to be influenced by the thyroid status during both of its stages (i.e., embryonic development and maturation), directly by altering kidney structure, glomerular function, tubular absorptive, and secretory capacities of the electrolyte channels, and indirectly influencing the renal blood flow via its effects on the cardiovascular system.
In another micropuncture study, proximal tubules of thyroidectomised rats showed a more than 65% increase in isotonic fluid reabsorption after treatment with T3.16,17 Studies carried out in rats and humans indicated that there was an increase in eGFR by up to 18% due to hyperthyroidism,13,18 while some studies also showed hyperthyroidism to cause decrease in serum creatinine and an increase in serum cystatin C.19 Inversely, the decline in eGFR was commonly seen in patients with subclinical or overt hypothyroidism.13,18 This analysis demonstrated a linear relationship between T3 and eGFR, indicating the fact that T3 decreased with declining eGFR, which has been linked and attributed to a reduction in the peripheral synthesis of T3 from T4. Such change in thyroid hormone balance could be attributed to a self-protecting mechanism in cases of severe levels of disease to diminish the metabolic requirement, the underlying mechanisms of which still remain unknown. Some studies predicted a higher prevalence of ESS or hypothyroidism in the CKD population.4 Song et al.4 demonstrated an increasing trend of low T3 population in accordance to the increasing CKD stage with normal TSH levels (eGFR <15: 78.6%; 15 ≤eGFR <30: 60.6%; 30 ≤eGFR <60: 20.8%; 60 ≤eGFR <90: 10.9%; eGFR ≥90: 8.2%).4 Similarly, in the author’s analysis, a large proportion of patients with Stage 4 and 5 CKD were also found to have ESS (32%). Meanwhile, the low T3 syndrome has become a marker of severe disease.20 Carrero et al.21 indicated, due to its intimate association with inflammation, the low T3 levels to be independent predictors of cardiovascular disease and all-cause mortality in patients with ESS.
Although this study did not test for inflammatory markers in the Stage 4 and 5 CKD population, it did show a high prevalence of hypothyroidism in these patients. Therefore, higher levels of inflammation in patients with Stage 4 and 5 CKD induce an increased frequency of euthyroid. Case reports, pilot studies, and meta-analyses carried out in humans have documented worsening levels of serum creatinine with hypothyroidism.22-25 Similar to the above-mentioned reports, a big proportion of the author’s patients with Stage 4 and 5 CKD also had hypothyroidism (18%), with significant predominance in the Stage 5 group. Understanding the impact thyroid dysfunction has on kidney functions and its importance have been highlighted in recent studies, which indicate clinical and subclinical hypothyroidism to be common in patients with eGFR <60 mL/min per 1.73 m2, leading to the question of whether hypothyroidism could be one of the contributing factors to a lower eGFR in some of these individuals.3 Hypothyroidism has been attributed to patients with serum creatinine levels exceeding 6 mg/dL, with a few even being described to have end-stage kidney disease.26
Many reviews have tried to establish or illustrate the interplay between the organs, and to establish the cause and effect relationship between the thyroid gland and the kidneys, or vice versa. Echterdiek et al.27 indicated in their review the positive effect of treating subclinical hypothyroidism in paediatric patients who are non-dialysis dependent and dialysis dependent.27 While mortality analysis was not conducted in this review, high TSH levels in patients with Stage 4 and 5 CKD had a higher mortality rate compared to normal TSH levels.28 Although data analysing the use of thyroid hormone supplementation and its outcomes in the patients with kidney disease are scant, a recent analysis carried out in veterans in the USA with Stage 3 CKD showed that patients with undertreated and untreated hypothyroidism have a higher mortality risk compared with those with euthyroid, whereas veterans who were treated to target hypothyroid had a better survival.29 Patients with CKD, when compared with the general population, have a higher incidence of cardiovascular deaths even in early stages, which increases significantly with declining or worsening of renal function. This causes a higher mortality, which increases many times in the presence of thyroid dysfunction, the pathologic basis of which are not only the haemodynamic changes, but also due to the increased endothelial damage and vascular calcification in conjunction with atherosclerosis. Atherosclerosis is also accelerated due to the presence of dyslipidaemia, causing alterations in the ion channel expression of the cardiac myocytes, leading to QT interval prolongation which heightens the risk of arrhythmias and sudden cardiac death in these patients.30-35
CONCLUSION
Of the patients with Stage 4 and 5 CKD, 68% had some kind of thyroid dysfunction. Hypothyroidism had an increased prevalence in patients with CKD. Patients with low T3 and T4 syndrome progressively increased as the severity of chronic kidney disease increased. eGFR negatively correlated with T3 levels, and positively with TSH. Thyroid dysfunction had an increased prevalence in patients with CKD in the age group of 31–60 years, but did not show any increased incidence in either male or female patients with CKD.
LIMITATIONS
This study is not without its limitations, some of which have been highlighted here. The patient pool was comprised only of patients with Stage 4 and 5 CKD, none of whom were undergoing dialysis, as it impacts the thyroid profile of these patients due to metabolism, systemic acidosis, chronic inflammation, etc. A small sample size was used, which included a relatively older population that may have significant impact on thyroid panels. A single centre study often has a crowd bias; however, this analysis included patients with CKD irrespective of aetiologies, thereby providing believable results. Lastly, as happens with all observational analysis, it is difficult to exclude all confounding factors and establish a causal relationship.
FUTURE RESEARCH
Prospective analysis is necessary to determine the influence supplementation of thyroid hormone has in patients with high TSH levels, either subclinical or overt hypothyroidism, in the disease progression and mortality for patients with Stage 4 and 5 CKD.








