Abstract
Acute kidney injury (AKI) in tropical countries is strikingly different from that in countries with a temperate climate. Tropical regions are characterised by year-round high temperatures and the absence of frost, which supports the propagation of infections that can potentially cause AKI. The aetiology and presentation of AKI reflects the ethnicity, socioeconomic factors, and ecological conditions in tropical countries. Apart from infections, other causes of AKI include exposure to animal toxins, ingestion of plant toxins or chemicals, poisoning, and obstetric complications. The low income status, poor access to treatment, and sociocultural practices (use of indigenous medicines) contribute to poor outcomes of patients with AKI. The exact aetiologic diagnosis often cannot be made due to lack of appropriate laboratory services. The epidemiology of AKI in tropical regions is changing over time. Renal replacement therapy is inaccessible to the majority and late presentation with delayed treatment add to the risk for future development of chronic kidney disease. AKI is often the primary cause of chronic kidney disease in the developing world, which increases demand for renal replacement therapy and transplantation. Most causes of AKI in developing countries are preventable and strategies to improve the public health and increased access to effective medical care are the need of the hour. This review offers comprehensive ideas about epidemiology, aetio-pathogenesis, clinical presentation, diagnosis, treatment, and prevention of community-acquired AKI in the tropics, with special reference to the Indian subcontinent. AKI is an under-recognised cause of morbidity and mortality in developing countries and even small, simple interventions could have an impact on its outcome.
INTRODUCTION
The lack of a uniform single definition, absence of multicentre studies, and under-reporting of acute kidney injury (AKI) in the tropics has led to difficulties in defining true epidemiology. The most recent definition of AKI proposed by the Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guidelines workgroup retains the Acute Kidney Injury Network (AKIN) and Risk, Injury, Failure, Loss, and End-stage kidney disease (RIFLE) staging criteria1,2 (Table 1). In the developed temperate countries, AKI usually occurs as a part of multi-organ involvement, especially in elderly patients; while in tropical countries it is usually community acquired, affecting the younger individuals.3 The published data on AKI incidence in developing countries are scarce4,5 and the risk of progression to chronic kidney disease (CKD) is unknown.6
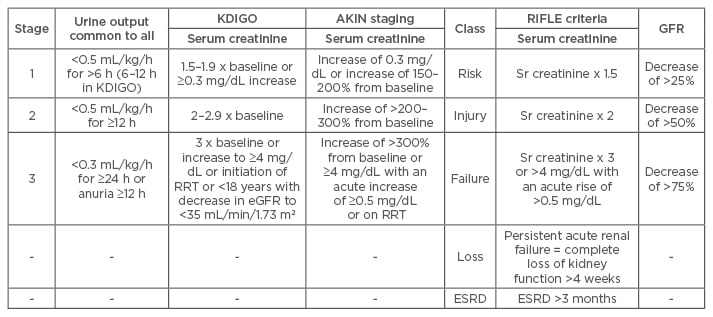
Table 1: Acute kidney injury classification and staging.
AKI: acute kidney injury; eGFR: estimated glomerular filtration rate; RRT: renal replacement therapy; ESRD: end-stage renal disease; KDIGO: Kidney Disease: Improving Global Outcomes; AKIN: Acute Kidney Injury Network; RIFLE: Risk, Injury, Failure, Loss, and End-stage kidney disease.
EPIDEMIOLOGY
The epidemiology of AKI is largely influenced by environmental factors and climatic conditions in tropical regions. Most published data are from single-centre studies conducted in urban areas and might not reflect the true prevalence of AKI. Approximately 0.1–0.25% of all admissions or 6.6 out of 1,000 admissions were for the management of AKI.7,8 The population of patients with AKI in developing tropical countries is younger (30–40 years of age) than that reported in developed temperate countries (60–70 years of age).9 The incidence of AKI is usually about 5–9% inwards and 30–36% in intensive care units. The incidence of AKI was predominantly seen during the months of July–September.
AETIO-PATHOGENESIS, DIAGNOSIS, AND TREATMENT
The causes of AKI in tropical countries can be due to infections, animal and plant toxins, drugs/ poisons, or obstetric complications.10 There are some newly emerging and rare causes for AKI that we commonly encounter. Figure 1 gives the overall aetiology, pathomechanisms, renal histology, and outcome of AKI in the tropics. Table 2 gives the detail regarding diagnostic tests, treatment, and outcome of common aetiologies of AKI.
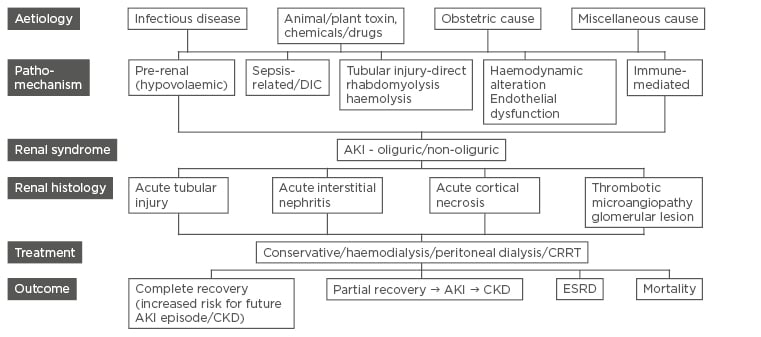
Figure 1: Schematic chart of pathomechanisms, renal histology, and outcome of various aetiologies of acute kidney injury.
AKI: acute kidney injury; CKD: chronic kidney disease; ESRD: end-stage renal disease; CRRT: continuous renal replacement therapy; DIC: disseminated intravascular coagulation.
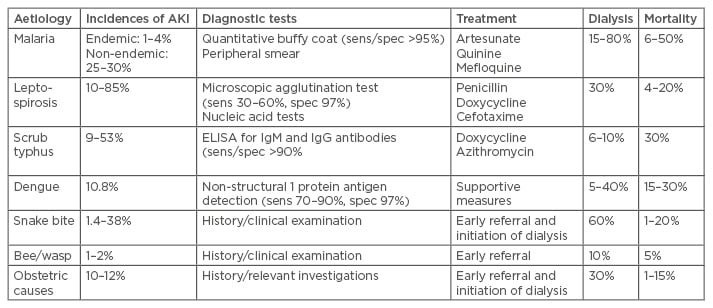
Table 2: Incidence, diagnostic tests, treatment, and outcomes of common causes of acute kidney injury.
AKI: acute kidney injury; Ig: immunoglobulin; ELISA: enzyme-linked immunosorbent assay.
Infection
The usual presentation of tropical febrile illness is fever with headache, severe myalgia, jaundice, intravascular haemolysis, and thrombocytopenia to variable extent.11 However, the similarity of the clinical presentations of the various forms of infection-associated AKI causes difficulty in diagnosis based only on clinical grounds.
Malaria
Most cases of malaria-associated AKI occur due to Plasmodium falciparum infection, although a few cases with Plasmodium vivax infection have been reported. About 50% of confirmed cases reported annually in India were due to P. falciparum. Severe parasitaemia, children aged <5 years, pregnant women, and immunosuppressed individuals have an increased risk of AKI.12 The pathomechanisms include cytoadherence of parasitised erythrocytes to the vascular endothelium, sequestration and obstruction of small vessels, immune-mediated glomerular and tubular damage, alterations of renal haemodynamics, and proinflammatory monocyte activation with release of tumour necrosis factor-α.13 The risk factors for high mortality include late referral, high parasitaemia, oliguria, hypotension, multisystem involvement, hepatitis, or acute respiratory distress.14,15
In a study involving 59 patients, P. falciparum malaria was seen in 76.3%, P. vivax in 16.9%, and mixed infection in 6.8% of patients. Dialysis was undertaken in 92% of patients, 81.3% of patients had complete renal recovery, 11.8% succumbed to malaria, and 6.9% progressed to CKD.16
Leptospirosis
Leptospirosis is a zoonosis caused by Spirochete leptospira. There are >200 pathogenic serovars with rodents as major reservoirs.17 Classic leptospirosis consists of two phases: the initial lepto-spiraemic phase lasting 4–7 days followed by the immune phase, characterised by an increase in immunoglobulin M (IgM) antibodies, and the development of disease manifestations in various organ systems.18 The pathogenesis includes direct nephrotoxicity, hyperbilirubinaemia, rhabdomyolysis, and hypovolaemia.19 The major histological findings are acute interstitial nephritis (AIN) and acute tubular injury (ATI).20 The leptospirosis laboratory, first established in July 1994 at the Institute of Nephrology, Madras Medical College, Chennai, India, is where microscopic agglutination tests, IgM enzyme-linked immunosorbent assay (ELISA), and macroscopic slide agglutination tests are carried out.21 In the recent past, leptospirosis-associated AKI at the centre had significantly declined from 31% in 1987– 1991 to 7.5% in 1995–2004 due to greater awareness of disease, availability of better diagnostic facilities, and widespread use of antibiotics. Faine had evolved criteria for diagnosis of leptospirosis on the basis of clinical, epidemiological, and laboratory data (World Health Organization [WHO] guidelines).22 Certain necessary modifications had been set (Modified Faine’s Criteria) to make the diagnosis early and simple.23 A 2007 study from Sri Lanka followed-up 44 patients with leptospirosis-associated AKI for at least 1-year, of which 9% showed progression to CKD. 24
Scrub typhus
Scrub typhus is caused by an obligate, intracellular gram-negative bacterium Orientia tsutsugamushi which is maintained by transovarian transmission in trombiculid mites.25 It presents with fever, eschar at the site of mite bite, maculopapular rash, and multiorgan involvement (interstitial pneumonia, meningitis, and hepatitis). Prerenal azotaemia is the main cause of AKI, though direct invasion of bacterium, systemic vasculitis, interstitial nephritis, and pigment nephropathy due to rhabdomyolysis can occur.26-28 In a study of 35 children with scrub typhus, an eschar was observed in 11%. Complications included myocarditis with cardiogenic shock in 34% and AKI in 20%.29 Creatine phosphokinase was raised in 55% and haemodialysis was undertaken in 10%. Independent predictors of AKI were intensive care unit requirement and thrombocytopenia.30 Renal failure was found to be an independent predictor of mortality.31
Dengue
Dengue is caused by an arbovirus, transmitted by Aedes aegypti female mosquitoes and about 40% of the world’s population are living in areas of increased risk of dengue infection. In addition to climatic conditions, uncontrolled and unplanned urbanisation, and migration of people are important factors for epidemics of dengue.32 The spectrum of dengue infection may be asymptomatic or classic dengue symptoms, such as fever, headache, retro-ocular pain, myalgia, arthralgia, and skin rash or dengue haemorrhagic fever (DHF), or dengue shock syndrome (DSS). AKI is an unusual complication of dengue, frequently associated with hypotension, rhabdomyolysis, or haemolysis, immune complex mediated-acute glomerulonephritis, or sepsis. The indicators of mortality were severe dengue, oliguria AKI, respiratory failure, and prolonged prothrombin or activated partial thromboplastin greater than twice the normal values. Lee et al.33 reported a 4.9% incidence of AKI in 81 patients suffering from DHF/DSS. Based on the AKIN criteria, the results revealed that 5.4% had mild AKI, 3.1% had moderate AKI, and 2.2% had severe AKI.34 Mallhi et al.35 reported a high incidence of AKI (14.2%) among dengue patients with mortality rates of 1.2%.
HIV
In patients with HIV, AKI occurs with sepsis, hypotension, dehydration, exposure to nephrotoxins, and toxic effects of antiretroviral therapy (ART).36 The most common nephrotoxic effects associated with ART include crystal-induced obstruction, mainly indinavir and atazanavir, and proximal tubule damage related to tenofovir. The combination of tenofovir with didanosine should be avoided because of its potentially addictive toxicity. Severe immunosuppression (CD4+ count <200 cells/mm3 and HIV RNA level >10,000 copies/mL) means a greater risk of AKI development.37,38 AKI was noted in 138 (3.9%) patients in a study. Hypovolaemia (44.2%) and sepsis (14.5%) contributed to AKI in the majority of cases. Acute tubular necrosis (ATN) was the most common histology, followed by AIN and diffuse endocapillary proliferative glomerulonephritis. In hospital mortality was 24.64%. A lower CD4 count decreased serum albumin levels, and Stage 4 WHO disease were associated with higher mortality.
At 3 months, complete recovery of renal function, CKD Stages 3–5, and progression to end-stage renal disease (ESRD) were noted in 58.69%, 14.5%, and 2.2% of cases, respectively.39 Rarely, thrombotic microangiopathy (TMA) caused by HIV infection can present with AKI.40 Acute diarrhoeal disease
AKI secondary to acute diarrhoeal disease (ADD) is still a major health problem in rural areas of tropical countries, though the dialysis requirement of ADDassociated AKI has recently decreased to 17% in the paediatric population.41 Rotavirus infection is the most common cause followed by Escherichia coli, Vibrio cholerae, Shigella, or Salmonella.42 However, the mortality rate associated with diarrhoea-related AKI remains high. The mortality rate was 2.1 in 1,000 admissions in a study conducted by Kumar et al.43
Acute Pyelonephritis
Renal infection can range from mild acute pyelonephritis (APN) to renal abscesses or emphysematous pyelonephritis (EPN).44 APN and EPN were seen in 75.2% and 24.7% of patients, respectively. E. coli was the most common organism. The mortality rate was 12.4% and the presence of shock and altered sensorium were associated with poor outcome in patients.45 It was found that about 121 patients were admitted with pyelonephritis in 22 months in our institute; about 67.7% were diabetics and 42.9% were in AKIN Stage 3 at presentation. Bilateral involvement was present in 55 patients (45%) and EPN was seen in 11 patients (9%). E. coli (27%) was the commonest organism, followed by Klebsiella. Around 60 patients were supported with renal replacement therapy (RRT). The mortality rate was alarmingly high at about 11% (unpublished data).
Drugs and Native Medications
Easy access to over-the-counter medications and dispensing drugs, including antibiotics, without proper prescriptions results in increasing incidence of AKI due to drug use. The common drugs that cause AKI include angiotensin-converting enzyme inhibitors, non-steroidal anti-inflammatory drugs, amino glycosides, anti-cancer, and lithium.46 Indigenous medical systems based on remedies derived from local plants and animals flourish in tropical countries. Anti-tuberculous treatment also contributes to drug-induced AKI by causing direct tubule-toxicity.47 Potentially toxic substances, such as paint thinners, turpentine, copper sulphate, and potassium permanganate, added to the plant extracts to increase their effect, cause AKI. The kidney is the usual route of excretion; the high blood flow rate and large endothelial surface area of the kidneys results in high concentration in the medulla resulting in AKI. Recently, there have been an increasing number of AKI reports among young people who are amateur body builders in tropical countries due to the use of creatine supplements, vitamin D overdosage, and anabolic steroids.48
Chemical Toxins
Accidental, intentional, or occupational exposure to chemicals that induce AKI is well documented in tropical regions. AKI occurs after the ingestion of chemicals, such as dimethyl bipyridinium dichloride and organo-phosphorus containing pesticides, copper sulphate, and mercuric chloride for suicidal purposes. There is a rise in AKI due to paraquat poisoning.49,50 We reported a patient who consumed mercuric chloride used for folk remedies with suicidal intent, and presented with AKI, gastrointestinal erosion, and disseminated intravascular coagulation.51 Diethylene glycol is present in brake oil and is used as an illegal adulterant in ethanol spirits or in medications. Ingestion of brake oil causes acute abdomen, dialysis-requiring renal failure, hypertension, deafness, and multiple neurological deficits due to poisoning.52 We published our experience of 32 cases that developed AKI in a toxicology unit.53 The risk of developing AKI was greater among the poisoning caused by bites and stings (6.15%) than by chemical poisoning (0.9%). Copper sulphate and rat killer poisonings were the most common causes of chemical-induced AKI.
Plant Toxins
Starfruit (Averrhoa carambola) juice is popular in India as an indigenous medicine and fruit drink, and contains a high oxalate concentration. Taking this fruit juice in large quantities on an empty stomach and in a dehydrated state causes nephrotoxicity. In a case series of five patients, all became symptomatic 10–12 hours after eating and developed AKI. Renal biopsy revealed ATN and all improved with complete renal recovery.54 Consumption of uncooked beans from the djenkol plant, especially in individuals with a low fluid intake, can cause dysuria, lumbar pain, hypertension, haematuria, and oliguria secondary to the intratubular formation of djenkolic acid crystals.
Animal Toxins
Snake envenomation is the most common cause of animal toxin-induced AKI. Stinging insects, such as honeybees, wasps, yellow jackets, and hornets are also common in tropical regions.
Snake bite
Of the 2,000 species of snake found worldwide, 450 are venomous. The highest number of snakebite-related deaths, about 45,900 every year, is reported in India. Onset of AKI can occur within 4–6 hours after the bite, or may be delayed for 3–4 days. The pathogenic mechanisms involved are haemodynamic alterations induced by cytokines and vasoactive mediators leading to renal ischaemia, haemolysis, disseminated intravascular coagulation, rhabdomyolysis, and direct nephrotoxicity caused by metalloproteases and phospholipase A in the snake venom. Renal biopsy showed ATN with pigment casts in 70–80% of patients.55 Other changes include TMA, mesangiolysis, interstitial inflammation, glomerulonephritis, vasculitis, and renal infarction.
In about 115 cases (1990–2014) of AKI, RRT was required in 106 (92.17%) patients. Complete recovery was seen in 51.30%, 13.04% expired during acute phase of illness, 3.47% developed CKD, and 9.56% required dialysis beyond 90 days.56 Prolonged snake bite to hospital time, hypotension, albuminuria, and raised bleeding time and prothrombin time were significant independent predictors of AKI.
Bees and wasps
Bees and wasps can cause human injury by allergic reactions, occurring after one or several stings, or direct envenomation when a massive attack with hundreds or thousands of stings occurs. We published a case series of 11 patients with wasp sting. All patients had evidence of rhabdomyolysis and three among them also had haemolysis. Ten patients required haemodialysis with a mean haemodialysis session of 8.7±2.8. Renal biopsy was carried out in 4 patients, which showed AIN in 1 patient, ATN in 2, and 1 patient had both AIN and ATN. Two patients with AIN were given steroids, others were managed with supportive measures. 1 patient died within 48 hours of presentation due to shock. At mean follow-up of 24 months, 1 patient progressed to CKD.57
Obstetric
After legalisation of abortion and improvements in antenatal care, incidence of obstetric AKI in India decreased from 22% in the 1970s to about 8% in the 1990s. Pregnancy-related AKI (PRAKI) however, continues to be prevalent and has a poor prognosis in India even today. We prospectively studied 130 patients (2010–2014) with PRAKI.58 The mean age was 25.4±4.73 years. The incidence of AKI in pregnancy was 7.8%. The aetiology was sepsis (39%), pre-eclampsia (21%), placental abruption (10%), ADD complicating pregnancy (10%), TMA (9%), postpartum haemorrhage (2%), and glomerular diseases (9%). Renal biopsy carried out in 46 patients showed renal cortical necrosis (n=16), TMA (n=11), ATI (n=9), AIN (n=1), and glomerular disease (n=9). Thirty-four patients were managed conservatively while 96 required dialysis. Complete recovery occurred in 56% and about 36% had persistent renal failure at 3 months. The mortality rate observed was 8%. Low mean platelet count, higher peak serum creatinine, dialysis dependency at presentation, and histopathologically presence of cortical necrosis and TMA predicted the progression to CKD.
Miscellaneous Causes
Post-infectious glomerulonephritis,59 rapidly progressive glomerulonephritis, and natural disasters are various other causes of AKI in the tropics. Liver disease-related AKI is on a rise. In total, 87 patients were included in 1-year, of which 85 were male. Hepatorenal syndrome was seen in 37 patients, sepsis in 25, hypovolaemia in 20, and other causes (native medicine and IgA nephropathy) in 5 (unpublished data). The raw gallbladder freshwater carp/grass carp are used for medicinal purposes in rural areas of India. Acute hepatic failure and AKI has been reported after consumption of these items. AKI with oliguria develops within 48 hours and lasts 2–3 weeks.
RENAL REPLACEMENT THERAPY
The choice of RRT can be intermittent haemodialysis, continuous RRT (CRRT) and acute peritoneal dialysis according to the availability and haemodynamic stability of patients. The indications include uraemic symptoms, symptomatic volume overload, uraemic pericarditis, severe metabolic acidosis, and hyperkalaemia. The clearance of urea and other molecular waste products is much faster with haemodialysis compared to peritoneal dialysis, but peritoneal dialysis does not need a special set-up as it can be started immediately and can be utilised in the case of haemodynamic instability.60
FOLLOW-UP, OUTCOME, AND PREVENTION
AKI is independently associated with new onset CKD, ESRD, cardiovascular disease, and all-cause mortality.61 The risk of CKD is exacerbated by the late presentation of AKI. Among patients who survive an episode of AKI, 10% had developed ESRD at 3.0–3.5 years.
Studies suggest that patients who receive followup by a nephrologist after an episode of AKI have improved outcomes compared with patients who did not.62 Only a minority of patients come for follow-up after an episode of AKI in the developing world, and the optimal strategies to promote rehabilitation after AKI are not well-defined. A retrospective study showed that 15% of AKI survivors were discharged on RRT, 12.5% remained dialysis-dependent, and 19–31% had CKD at longterm follow-up.63 We urge healthcare providers to consider intensive follow-up for every patient who survives an episode of AKI. Establishing national and regional registries for data collection, processing, and reporting data availability will confer public and political prominence on AKI.
Interventions such as lifestyle changes, medication reconciliation, blood pressure control, and education could have significant populationlevel effects, and promote renal recovery. More research is needed to identify the highest-risk patients with AKI and to determine the components of evidence-based rehabilitation after AKI. An integrated approach to reduce the disease burden, requires a change in public policy and a change in focus away from hospital-based care and towards improvement of basic health needs. Vector control is the most effective way to prevent transmission of vector-borne-diseases. Public awareness of the need for use of safe water, safe handling, and use of pesticides and other nephrotoxins should be increased.
OUR DATA
Between January–December 2016, 1,003 patients were admitted to Rajiv Gandhi Government General Hospital, Chennai, India, with AKI (unpublished data). In respect to AKIN staging, 91 patients were in Stage 1, 271 in Stage 2, and 644 in Stage 3. Medical causes constituted 89%, surgical 7.6%, and obstetric 3.4%. Of the medical causes, sepsis was the most common (19.5%) followed by glomerular disease (10.7%), tropical febrile illness (9.14%), liver disease-related (8.64%), snake bite (6.6%), malignancy-related (6%), and acute poisoning (5.3%). Around 532 were managed conservatively, 367 received haemodialysis, and 119 received peritoneal dialysis. Of these patients, 70.4% improved with therapy, dialysis dependence was seen in 3.3%, 5.2% patients discharged against medical advice, and 20.8% died. Compared to our previous data,64,65 the incidence of AKI due to sepsis (especially urosepsis), liver disease, and scrub typhus is increasing while there is a decline in the incidence of AKI due to leptospirosis, malaria, ADD, and obstetric causes similar to other published data. Mortality is also relatively low.
Comparison of Acute Kidney Injury Between Developing Tropical and Developed Nations
It is estimated that 85% of AKI episodes occur in developing countries with a tremendous impact on their public health. The male-to-female patient ratio of AKI in the developed world is close to 1:1 but is skewed in developing countries to between 1.8:1 and 5:1. In the developed world, intrinsic renal disease due to shock and sepsis predominates as a cause of AKI while volume-responsive.66 In the developing world, RRT is often only available in large cities, with cost and difficulties in transportation being the main limitations.67 In spite of marked improvements in the care of critically ill patients with AKI, mortality remains high in developed countries (40–70%), but mortality in developing countries seems to be lower, ranging between 10% and 40%.68 As effective therapies are less accessible and with resource limitations, early preventive and therapeutic measures are essential to decrease mortality and cost. The major difference in AKI in tropical countries is due to poor health awareness and sanitation, overcrowding, unavailable/deficient prompt diagnostic tests, poor healthcare, less availability of dialysis resources, and professional experts. This may be improved by health education and awareness that has to be incorporated in school education, educating the public through media, increasing the GDP spent on health by political commitment, strengthening primary and referral health sectors, and finally to create awareness in the general public of the importance of seeking treatment early.
CONCLUSION
Community-acquired AKI is a major health problem in tropical countries. Need for intensive care and multisystem involvement carries poor prognosis. Recovery of renal function after an episode of AKI is an important determinant of further survival and life expectancy. The long-term renal prognosis is usually poor in AKI survivors. Timely referral of patients and early initiation of treatment will improve outcomes.








