Presenters: Eric W. Young,1 Philipp Csomor,2 Joel Gunnarsson,3 George Fadda4
1. Arbor Research Collaborative for Health, Ann Arbor, Michigan, USA
2. Vifor Pharma, Glattbrugg, Switzerland
3. Quantify Research, Stockholm, Sweden
4. California Institute of Renal Research, San Diego, California, USA
Disclosure: The analysis presented by Dr Young was funded in part by Vifor Pharma. Dr Csomor is employed by Vifor Pharma, and the study he presented was supported by Vifor Pharma. Mr Gunnarsson is employed by Quantify Research and the study presented was supported by Vifor Pharma. Dr Fadda has declared no conflicts of interest and the study he presented was sponsored by OPKO Health Renal Division.
Acknowledgements: Medical writing assistance was provided by Dr Juliet George, Chester, UK. The presenters were not involved in the writing or reviewing of this article.
Support: The publication of this article was supported by Vifor Pharma and reviewed by OPKO Health Inc.
Citation: EMJ Nephrol. 2020;8[Suppl 3]:2-10.
Summary
Secondary hyperparathyroidism (SHPT) is a frequent early complication of chronic kidney disease (CKD). At four international congresses in 2019 and 2020, experts Dr Eric W. Young, Dr Philipp Csomor, Joel Gunnarsson, and Dr George Fadda highlighted current issues in the management of SHPT and presented new analyses and data concerning the efficacy and safety of available treatment options. Young et al.1 illustrated the importance of controlling parathyroid hormone (PTH) levels prior to dialysis, showing that predialysis levels of serum PTH influence both subsequent disease management and the risk of uncontrolled PTH during dialysis in patients with CKD. However, evidence from meta-analyses by Csomor et al.2 and Gunnarsson et al.3 revealed notable unmet needs in the management of PTH levels with existing therapies. The analyses confirmed an increased risk of hypercalcaemia with active vitamin D (AVD) analogue treatment in patients with non-dialysis CKD and elevated PTH, and findings presented by Gunnarsson et al.3 suggested that treatment with nutritional vitamin D (NVD) is not efficacious in reliably and consistently lowering PTH levels in patients with non-dialysis CKD and SHPT. Another treatment option, an extended-release formulation of the vitamin D prohormone calcifediol, has demonstrated safety and efficacy for the treatment of SHPT in non-dialysis CKD in randomised controlled trials (RCT). In the final poster reviewed here, Fadda et al.4 presented encouraging real-world data confirming the clinical efficacy and safety of this novel treatment option.
Introduction
SHPT is a common and serious complication of CKD that manifests early in the disease course.5 SHPT is characterised by excessive secretion of PTH and parathyroid hyperplasia, linked to abnormalities in calcium, phosphorus, and vitamin D levels.5,6 Left untreated, the prolonged elevation of PTH in SHPT increases the risk of bone disease, cardiovascular disease (e.g., vascular calcification), morbidity, and mortality in patients with CKD.5,7-9 Parathyroid gland hyperplasia leads to increased autonomy of parathyroid tissues, resulting in sustained elevations in PTH, and is accompanied by treatment resistance as a result of reductions in sensitivity to calcium and vitamin D hormone signalling.10 Low levels of vitamin D are central to the development and progression of SHPT, and the use of AVD analogues and NVD supplements have been one approach to treatment.5,6 However, because of limited evidence of reliable efficacy and/or safety, the current Kidney Disease: Improving Global Outcomes (KDIGO) guidelines do not recommend the routine use of AVD analogues in patients with non-dialysis CKD (G3a–5) and, because there is a lack of studies of sufficient duration, the efficacy of NVD supplements is considered unproven.7
The posters reviewed in this article present new analyses concerning SHPT management, with a focus on non-dialysis CKD. These latest findings illustrate the challenges, unmet needs, and meaningful benefits of early treatment of elevated PTH levels in patients with non-dialysis CKD and SHPT.
Parathyroid Hormone Levels During Dialysis Reflect Predialysis Management of Secondary Hyperparathyroidism
Dr Eric Young presented this session at the 2019 American Society of Nephrology (ASN) Annual Meeting held in Washington, DC, USA, this study by Young et al.1 aimed to assess the extent to which predialysis management of SHPT influences the prescription of PTH-lowering therapies and the risk of uncontrolled PTH during the first year of dialysis in patients with end-stage renal disease. Based upon data from the international Dialysis Outcomes and Practice Patterns Study (DOPPS), the sample population included 5,683 patients from 15 countries who had been on dialysis for <120 days.
The authors examined the monthly prescription prevalence of AVD analogues and calcimimetics, stratified by predialysis PTH levels (Figure 1). The median PTH level prior to dialysis was 275 (interquartile range: 155–472) pg/mL, with 16% of patients having PTH >600 pg/mL at initiation. Descriptive analysis showed that the rate of medication during dialysis use was influenced by predialysis PTH levels. Patients who initiated dialysis with a higher serum PTH level were more likely to be prescribed AVD in the early months of dialysis than those with lower predialysis PTH levels. Approximately 65% of patients with predialysis PTH >600 pg/mL were prescribed AVD during the first month of dialysis, compared to approximately 35% of patients with PTH <150 pg/mL; this pattern persisted throughout the assessed 12-month period (Figure 1A). Patients with a high predialysis PTH level were also much more likely to initiate calcimimetic treatment during the first year of dialysis. As shown in Figure 1B, approximately 25% of patients with predialysis PTH >600 pg/mL were prescribed a calcimimetic at 12 months, compared to <5% of patients with PTH <150 pg/mL.
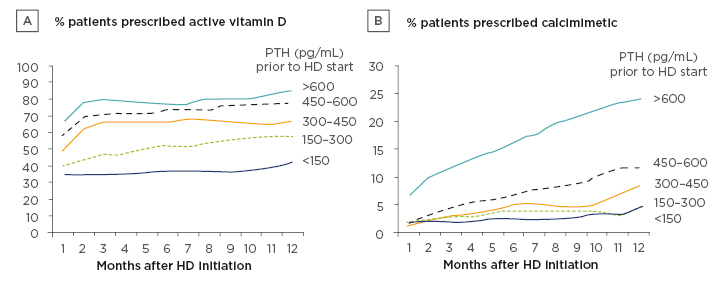
Figure 1: Parathyroid hormone-lowering medication prescriptions over the first year of dialysis, stratified by serum parathyroid hormone levels prior to initiation of dialysis.
HD: haemodialysis; PTH: parathyroid hormone.
Despite a more aggressive usage of these medications during dialysis, Young et al. found that high serum PTH levels prior to dialysis were strongly associated with high PTH serum levels 9 months after initiation of dialysis. There was a greater prevalence of high PTH (>600 pg/mL) after 9–12 months of dialysis in patients who initiated dialysis with a PTH level >600 pg/mL (29%) compared to patients with PTH levels 150–300 pg/mL at dialysis initiation (7%). This equated to a difference in absolute risk of 19% (95% confidence interval [CI]: 15.4–22.7%) after adjustment for potential confounding factors using linear probability models (Table 1).
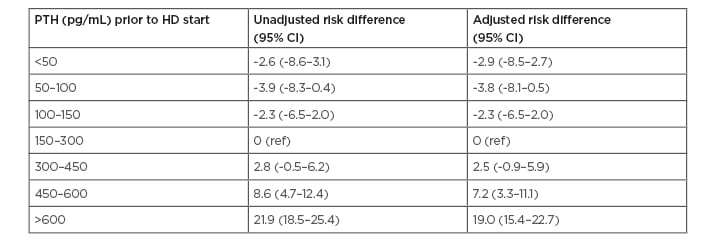
Table 1: Risk difference (95% confidence interval) of parathyroid hormone >600 pg/mL 9–12 months after dialysis initiation, by parathyroid hormone level prior to dialysis initiation.
Risk difference: adjusted difference in the probability (shown as percentage) of PTH >600 pg/mL at 9–12 months after dialysis initiation, estimated across levels of PTH measured immediately prior to dialysis initiation (reference: PTH 150–300 pg/mL immediately prior to dialysis initiation).
CI: confidence interval; HD: haemodialysis; PTH: parathyroid hormone; ref: reference.
Dr Young and his team concluded that high PTH levels during the first year of dialysis reflect, in part, suboptimal management of SHPT at the earlier predialysis stage. The findings were consistent with their initial hypothesis that predialysis levels of serum PTH influence both subsequent disease management and the risk of uncontrolled PTH during dialysis.
Active Vitamin D Analogues Increase the Risk of Hypercalcaemia in Patients with Secondary Hyperparathyroidism
As seen in the previous poster by Young et al., AVD analogues are routinely used to treat SHPT, and RCT have demonstrated their efficacy in suppressing PTH levels in patients with non-dialysis CKD.11,12 However, their efficacy is coupled with safety concerns, namely an increased risk of hypercalcaemia,7,11-13 and consequently, AVD analogues are not recommended for routine use in non-dialysis CKD by the updated 2017 KDIGO guidelines.7 This latest guidance was mainly based upon the raised rate of hypercalcaemia seen with paricalcitol (versus placebo) in the PRIMO and OPERA RCT.7,11,12 Csomor et al.2 performed a systematic review and meta-analysis to further evaluate the effect of AVD analogues on hypercalcaemia in patients with non-dialysis CKD and SHPT, the results of which were presented by Dr Philipp Csomor at the 2019 International Society for Pharmacoeconomics and Outcomes Research (ISPOR) Annual Conference held in Copenhagen, Denmark, and are reported here.
Six articles reporting RCT of patients with non-dialysis CKD and elevated PTH who received AVD analogues (≥30 patients per arm; duration ≥6 weeks), and which specified the rate of treatment-related hypercalcaemia, were eligible for inclusion. Five articles evaluated paricalcitol, and one evaluated alfacalcidol, involving a total of 799 patients.
Across the six studies, the frequency of hypercalcaemia events, classified as related or possibly related to the study drug, ranged from 1.1% to 43.3% in the AVD analogue-treated groups versus 0.0% to 3.4% in the placebo groups. After exclusion of one study (Fishbane et al.14) that had a ‘high risk’ of bias according to the Cochrane Collaboration criteria,15 meta-analysis showed that patients treated with an AVD analogue had a 7.2-fold greater risk of hypercalcaemia versus placebo (95% CI: 2.21–23.60; p=0.001). However, a sensitivity analysis that included all six studies also highlighted a significantly increased risk of hypercalcaemia with AVD analogue treatment (6.6-fold) compared to placebo (95% CI: 2.37–18.55; p<0.001) (Figure 2A).11,12,14,16-18
After evaluating study heterogeneity, the authors performed a secondary sensitivity analysis, which excluded the OPERA and PRIMO studies11,12 that accounted for a large number of the observed hypercalcaemia events. Even after these studies were excluded, the rate of hypercalcaemia was shown to be 3.0-fold greater in patients receiving AVD analogue treatment than in patients receiving placebo (95% CI: 1.06–8.71; p=0.039) (Figure 2B).14,16-18
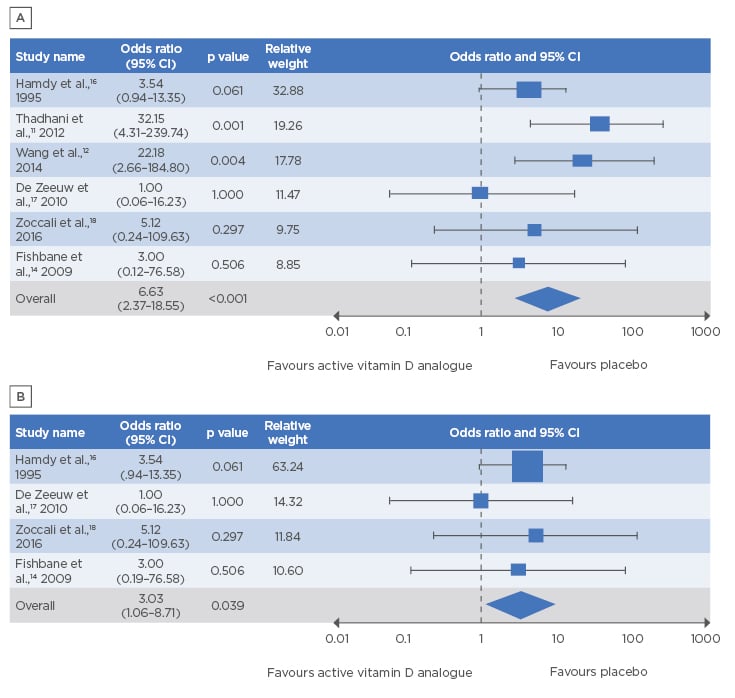
Figure 2: Forest plot showing a statistically significantly increased risk of hypercalcaemia with active vitamin D analogues versus placebo.
A) Sensitivity analysis (n=6 studies); B) secondary sensitivity analysis (n=4 studies).
CI: confidence interval.
Although limited by the small number of studies available, the meta-analysis by Csomor et al.2 confirmed the increased risk of hypercalcaemia with AVD analogue treatment in patients with non-dialysis CKD and elevated PTH (versus placebo), even with exclusion of data from the OPERA and PRIMO studies. The authors concluded that the undesirable elevations in serum calcium observed in this analysis highlight an urgent unmet need for new therapies for patients with non-dialysis CKD and SHPT.
Limited Evidence of the Efficacy of Nutritional Vitamin D Supplements in Reducing Parathyroid Hormone Levels
As well as AVD analogues, NVD supplements are frequently used to address SHPT in patients with CKD, particularly in patients with early disease. However, to date, evidence assessed from RCT on the PTH-lowering effects of NVD supplements has been inconsistent.7,19-21 To reassess the topic of NVD supplementation to reduce PTH levels in SHPT, Gunnarsson et al.3 performed a comprehensive meta-analysis to study the effects of the NVD cholecalciferol and ergocalciferol in controlling PTH and increasing 25-hydroxyvitamin D (25[OH]D) levels in patients with non-dialysis CKD, the results from which were presented by Joel Gunnarsson from 6th to 9th June 2020 at the European Renal Association – European Dialysis and Transplant Association (ERA-EDTA) Virtual Congress 2020.
Based on a broad systematic literature review of treatment alternatives in SHPT, this meta-analysis included RCT of adults with non-dialysis CKD (at least 20 patients per trial) that reported PTH and 25(OH)D outcomes. Individual study results were then pooled using a fixed-effects model with inverse variance weighting. The authors identified 14 eligible articles, all of which were used to assess the effects of NVD on PTH levels versus baseline. In addition, nine of these articles had a comparator arm, allowing investigation of the effects of NVD supplements versus placebo or no treatment. The studies included a total of 745 patients with an average baseline PTH level of 144 pg/mL and a 25(OH)D level of 19.62 ng/mL.
The meta-analysis revealed that NVD treatment produced only small changes in PTH levels from baseline, with an overall reduction of 10.53 pg/mL (95% CI: -16.33 to -4.72) observed across studies (Figure 3).20-33 There was a greater relative reduction in PTH levels when compared to placebo or no treatment (49.74 pg/mL; 95% CI: -70.17 to -29.3), which was driven by increases in PTH in the placebo/untreated patient groups rather than an active reduction of PTH by the NVD treatment. Based on the Grading of Recommendations Assessment, Development and Evaluation (GRADE) working group methodology, the authors rated this evidence on PTH levels as low-to-moderate quality.
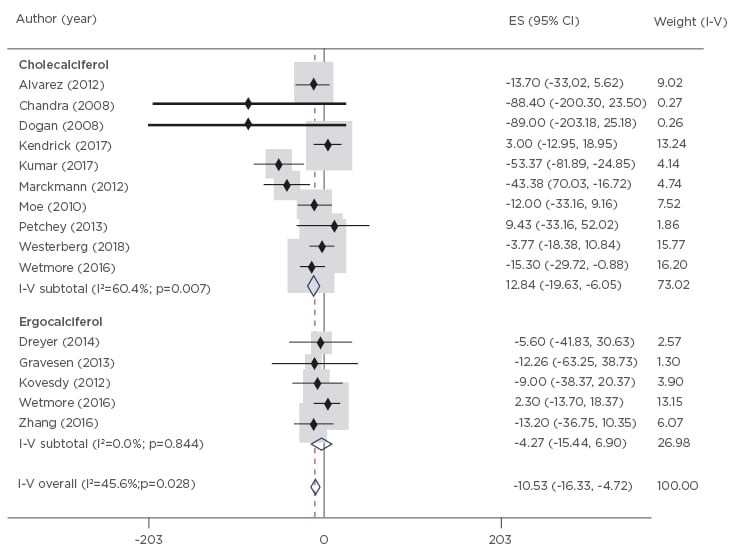
Figure 3: Changes in parathyroid hormone (pg/mL) from baseline to end-of-study in patients receiving nutritional vitamin D supplements.
CI: confidence interval; ES: effect size; I-V: inverse variance.
Examining changes in 25(OH)D levels with NVD treatment, Gunnarsson et al. found an overall increase of 20.62 ng/mL (95% CI: 19.58–21.65) from baseline, and a similar increase of 26.87 ng/mL (95% CI: 24.44–29.30) relative to placebo or untreated patients. However, the authors noted recent findings suggesting that levels of 25(OH)D need to reach >50 ng/mL to achieve clinically meaningful reductions in PTH and bone turnover markers.34 The present analysis found that average levels of 25(OH)D in treated patients reached >30 ng/mL in the majority of articles, but were >50 ng/mL in only five of the 14 articles. Thus, the authors concluded that there is limited potential for NVD treatment to increase 25(OH)D levels to a level (>50 ng/mL) that might be necessary for clinically meaningful PTH reductions. Moreover, the level of evidence on 25(OH)D levels with NVD treatment was graded as very low to low.
In summary, based on these meta-analysis findings, Mr Gunnarsson and his team suggested that treatment with NVD is not efficacious to reliably and consistently lower PTH levels in patients with non-dialysis CKD and SHPT.
Real-World Evidence Confirms the Efficacy and Safety of Extended-Release Calcifediol to Treat Secondary Hyperparathyroidism
As outlined by evidence from the previous posters, there is a need for treatments for SHPT that can be started early, and effectively and reliably reduce PTH without adversely impacting associated calcium and phosphorus levels. RCT have indicated that extended-release calcifediol (ERC) has a profile compatible with this unmet need; ERC has demonstrated safety and efficacy for the treatment of SHPT in adults with non-dialysis CKD (Stage 3 or 4).35,36 The study by Fadda et al.4 was the first to present real-world evidence on the effectiveness and safety of ERC; this poster was presented by Dr George Fadda from 26th to 29th March 2020 at the virtual National Kidney Foundation (NKF) Spring Clinical Meeting.
The study analysed data from 174 ERC-treated patients from 18 nephrology clinics across the USA during a 12-month follow-up period. Adult patients eligible for inclusion had CKD Stage 3 or 4, a history of SHPT and vitamin D insufficiency, and medical records available for >6 months post and prior to ERC initiation.
Patient demographics and characteristics of the real-world ERC cohort were similar to those of the RCT populations;35,36 the mean age was 69 years, 65% of patients were Caucasian, 52% were female, and there was an approximately equal distribution between CKD Stage 3 (47%) and Stage 4 (53%). Mean (standard error) levels of serum 25(OH)D, calcium, and phosphorus at baseline were 20.3 (0.7) ng/mL, 9.2 (0.1) mg/dL, and 3.8 (0.1) mg/dL, respectively, and also reflected levels seen in patients in the RCT. However, the authors noted that levels of serum PTH (181.4 pg/mL) in the real-world cohort were markedly higher than those observed in the RCT population (147.2 pg/mL).35
Examining serum levels post-treatment, Fadda et al. found that ERC had a significant effect in raising 25(OH)D levels by 23.7 (1.6) ng/mL to a mean of 44.0 (1.7) ng/mL, and decreasing PTH by 34.0 (6.6) pg/mL to a mean of 147.4 (7.2) pg/mL (both p<0.001 versus pretreatment values). Further analysis showed that 122 (70.1%) patients achieved serum 25(OH)D of ≥30 ng/mL, and 70 (40.2%) patients achieved ≥30% reduction in PTH. In terms of safety, ERC treatment had no statistically significant impact on serum calcium and phosphorus levels, which each showed a post-treatment change of only 0.1 mg/dL.
Comparing these real-world findings with those from the randomised studies by Sprague et al.,35,36 the authors reported that similar proportions of patients achieved the predetermined endpoints in the RCT at a similar length of follow-up: ≥30 ng/mL 25(OH)D (70.1% versus 80%/83% patients); ≥30% reduction in PTH (40.2% versus 33%/34% patients). They also noted that these real-world outcomes were achieved with the large majority of patients (98%) receiving ERC 30 μg/day, while in the RCT 74% of patients were force-titrated by protocol to ERC 60 μg/day after Week 12.35,36
In conclusion, the first real-world data on the effectiveness and safety of ERC are consistent with findings from RCT. The study by Fadda et al. confirms that ERC appears to fulfil an unmet need for the treatment of patients with non-dialysis CKD and SHPT, by producing a significant reduction in PTH, while avoiding adverse effects on key safety markers such as serum calcium and phosphorus levels.
Conclusions
There is an unmet need for an efficacious and safe treatment of SHPT in non-dialysis CKD patients. Evidence shows that treatment is required early in SHPT management, which could impact medication usage and reduce the risk of uncontrolled PTH during dialysis. Meta-analyses indicate that treatment with AVD analogues offers efficacy, but with notable safety concerns (hypercalcaemia), while NVD supplements appear safe, but provide limited efficacy. Reinforcing data from RCT and real-world evidence shows that ERC may address this unmet need by effectively reducing serum PTH, without impacting serum calcium, in patients with non-dialysis CKD and SHPT.

![EMJ Nephrology 8 [Supplement 3] 2020 Feature Image](https://www.emjreviews.com/wp-content/uploads/2020/10/EMJ-Nephrology-8-Supplement-3-2020-Feature-Image-940x572.jpg)






