Abstract
Acute kidney injury (AKI) is a major global health problem, occurring in >13 million people and responsible for >2.3 million deaths every year, 85% of which are in developing countries. Although the International Society of Nephrology (ISN) set a goal of eliminating preventable deaths by AKI by 2025, implementation of this program in developing countries presents major challenges for several reasons: there are few data on the epidemiology and causes of AKI in low- and middle-income countries (LMIC); health care resources to diagnose, manage, and treat AKI are often limited; and governments, institutions, and global health initiatives have not focussed sufficiently on the AKI problems. Thus, developing and implementing effective strategies to eliminate preventable deaths from AKI in LMIC have required efforts to better understand how to increase the awareness of AKI by health care workers and institutions.
INTRODUCTION
Acute kidney injury (AKI) is recognised worldwide as a major public health challenge, particularly in developing countries. Despite technological progress and preventive efforts, the AKI incidence remains high with >13 million cases globally per year, 85% of which are in developing countries, and can be attributed to >2.3 million deaths.1-4
Although AKI in high-income countries (HIC) with sophisticated medical infrastructure is predominantly a disease found in hospitalised, critically ill, and elderly patients, in low- and middle-income countries (LMIC), AKI is largely a community-acquired condition,1-9 with dehydration and hypotension appearing to be the most common causes, as seen in recently published data.10
In LMIC, several cases of community-acquired AKI are due to causes that have the potential to be reversed with simple interventions.5-10 Infectious diseases and obstetric complications are the leading causes of AKI, followed by animal venoms and natural herbal medicines. Although treating the underlying cause is of prime importance, death as a consequence of AKI may often be prevented by simple interventions such as oral rehydration or immediate temporary dialysis. However, in LMIC, acute renal replacement therapy (RRT) is available only in large cities, usually for the proportion of the population who can pay for treatment. Thus, patients who develop AKI and are in need of dialysis often die.3,9-13
Given the potential reversibility of AKI with early intervention, early diagnosis is of particular importance. Acute Dialysis Quality Initiative recommendations for diagnosis of AKI in LMIC11 include the estimation of urine output, measurement of serum creatinine levels by point-of-care tests (POCT), and thorough urinalysis. Dialysis might reduce mortality related to AKI in resource-limited settings. When RRT is available, peritoneal dialysis (PD) is used more commonly than intermittent haemodialysis, and these two techniques demonstrate equivalent outcomes.14
The International Society of Nephrology (ISN) AKI initiative aims to prevent all avoidable deaths by AKI by 2025 (0by25). In the context of the ISN 0by25 initiative, this report aims to revise the risk factors for AKI and its management challenges in low- and middle-income countries, focussing on barriers to early diagnosis and adequate treatment.
EPIDEMIOLOGY, AETIOLOGY AND RISK FACTORS
Despite the high burden of AKI,1,4,6-10 reliable information on AKI incidence in LMIC has been slow to be produced due to limitations in the quantity, quality, and availability of data. In the 2013 meta-analysis,2 nearly 84% of the included studies were from HIC, two studies were from Africa, and none were from Asia. In the 2015 meta-analysis,4 which included >77 million AKI patients and multiple reports from Africa and Asia, the AKI incidence in LMIC had increased and approached that seen in HIC: it ocurred in 21% of hospitalised patients, which is very similar to worldwide AKI incidence.2,4,7 However, the proportion of AKI patients requiring RRT in LMIC was lower than in HIC: 2% versus 11% of all AKI patients.
The aetiology of AKI in LMIC is often different from that seen in HIC and also differs between urban and rural areas. Rural, community-acquired AKI is associated with severe gastroenteritis; acute glomerulonephritis; envenomation; intoxication from traditional remedies; complications of endemic infections such as malaria, AIDS, leptospirosis, and dengue; obstetric complications including septic abortion; and use of nephrotoxic drugs and agentes. Conversely, aetiologies of AKI in large urban centres is similar to those in HIC2-4,10-11 (Table 1).
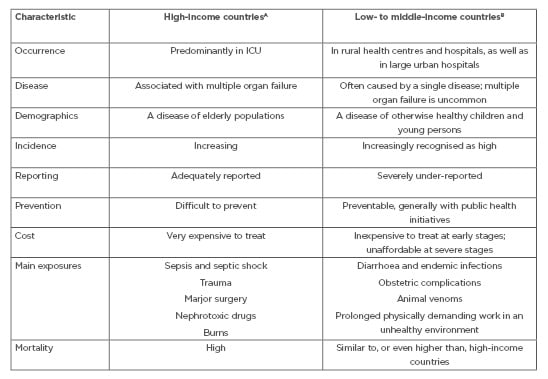
Table 1: The contrasting characteristics of acute kidney injury around the world.
ICU: intensive care unit.
A Includes World Bank upper-middle-income ($3,956–12,235 USD) and high-income (>$12,236 USD) categories.7
B Includes World Bank low-income (<$1,005 USD) and lower-middle-income ($1,006–3,955 USD) categories.7
The two most common causes of AKI in the 0by25 Global Snapshot10 were hypotension and dehydration occurring in 40% and 38% of patients, respectively. In this study, risk factors such as infection, sepsis, and use of nephrotoxic drugs were common to all countries. Several risk factors for AKI present in LMIC are modifiable, such as nephrotoxin exposure, dehydration, hypotension, anaemia. Other risk factors are non-modifiable, such as comorbidities (chronic kidney disease, diabetes, cancer and/or heart disease) and demographic factors (age, sex), and other factors are related to environmental and infrastructure risks such as inadequate sanitation, insufficient clean water, and inadequate control of parasites or infection carrying vectors. Nevertheless, the high incidence of community-acquired AKI caused by modifiable factors provides an opportunity to combat the problem at an early stage and consequently avoid disease progression.12,13
Given the limited health care investment in LMIC, which severely hinders the availability of RRT and intensive care, the focus in LMIC is to decrease AKI incidence by targeting modifiable risk factors, which are of importance in decreasing incidence, severity, and costs.
BARRIERS TO CARE: DIAGNOSIS AND TREATMENT
AKI has the potential to be treatable and reversible. However, early recognition failure is associated with its progression, which requires complex therapies, and leads to delayed or impaired recovery and high mortality.11,14 There are major challenges involved in developing strategies to establish an early AKI diagnosis and provide appropriate treatment in LMIC, such as the lack of laboratory supplies, necessary therapeutics, adequate medical infrastructure, and personnel.14,15 Additionally, limited education about kidney disease and inadequate access to basic health care in LMIC hinder the early recognition of AKI and delay intervention.2,14
Another barrier to AKI care regards the shortage of healthcare workers in areas such as Africa because of the brain drain to western countries. As a result, patients often spend days with worsening and untreated AKI.13 In sub-Saharan Africa, delays of up to 3 weeks between onset of symptoms and presentation to hospital were described in adults with AKI,13 whereas delays in presentation to hospital (mean 6 days) and treatment initiation were present in 50–80% of children with AKI.13
The problem of late AKI recognition has also been investigated by the 0by25 Global Snapshot.10 In LMIC, patients are rarely or never seen by a nephrologist and are rarely seen by a physician; rather, their first contact with the health care system occurs in the community dispensary, where unspecialist health care providers are the only available resource. Given the constraints, efforts to educate individuals about AKI in LMIC must focus on the most basic levels of the health care system.
Acute Dialysis Quality Initiative recommendations for diagnosis of AKI in LMIC11 include the estimation of urine output, measurement of serum creatinine levels by point-of-care tests (POCT), and thorough urinalysis. Recently, serum urea nitrogen levels, measured using a dipstick, has been studied in AKI; it is very cheap (<$1 USD) and the AUC was >0.75.16-18
To better understand the barriers to improving awareness of AKI in LMIC, a questionnaire was developed by a group of 20 nephrologists during the 2014 International Society of Peritoneal Dialysis Meeting in Madrid.14 These nephrologists included physicians from Africa, Asia, and North and South America. More than 80% of respondents indicated that the diagnosis of AKI in this setting is mostly based on clinical judgment, reflecting the limited availability of laboratory services. Only 60% of respondents indicated that these rural health centres have intravenous fluids and only 52% stated that they have appropriate antibiotic therapy to treat infection-related AKI. Antivenom therapy is generally not available in rural communities.18 All district health centres had oral rehydration solutions and 96% indicated that intravenous fluids and antimalarial drugs are readily available, 72% indicated that antibiotics are available, and 63% indicated that laboratory support is available to diagnose AKI. No respondents indicated that dialysis therapies were available.14
This shortcoming is highlighted in the study by Olowu et al.,13 which was performed in 13 countries in sub-Saharan Africa. In 3,340 patients admitted to hospital with AKI, the indications for dialysis was 66% in children and 70% in adults. However, only half of the children and one-third of adults received dialysis when required. In LMIC, RRT are available only in large cities, usually for the proportion of the population who can pay for treatment. Thus, patients who develop AKI and are in need of dialysis often die. Dialysis might reduce mortality related to AKI in resource-limited settings. When RRT is available, PD is used more commonly than intermittent haemodialysis, and these two techniques demonstrate equivalent outcomes.19-24 By contrast, gravity-driven PD is a more realistic option because RRT can be delivered without machines and electricity, relying only on consumable supplies, reducing costs and complexity in low resources settings.9,20 Although particularly useful in areas with fragile health infrastructure, PD is underused in most parts of the world, despite advantages such as reasonable costs (as little as $150 USD to save one life).24 This approach appears feasible, as documented by the encouraging results from PD programs for AKI in centres in Africa, Brazil, and Asia.5,19-27
THE SAVING YOUNG LIVES PROGRAM
The ISN 0by25 initiative, led by Ravindra Mehta and started in 2012,2 aims to eliminate or at least decrease preventable AKI-related deaths around the world by 2025, with a focus on low- and middle-income countries in Africa, Asia, and Latin America. Based on previous studies, avoidable deaths from AKI are known to happen as a result of three different situations: secondary public health problems such as diarrhea, endemic infections, and unclean water; late or no recognition; lack of access to laboratory studies or inadequate response to clinical treatment; and lack of acute RRT to treat life threatening fluid overload, acidosis, and hyperkalemia.2
Although knowledge of AKI epidemiology has greatly improved since the use of a standardised AKI classification system, few studies have focussed on community-acquired AKI in LMIC. In the meta-analysis by the 0by25 initiative, the main issues regarding AKI epidemiology were raised.2 Information was presented regarding the increasing associated mortality of even mild AKI, the effects of an AKI episode on long-term outcomes, and early detection and treatment of AKI in outpatient and low-resource settings. However, to reduce AKI related mortality and morbidity, knowledge of the factors that affect AKI outcomes is key to implementing initiatives.
The AKI meta-analysis published in 2015 by Mehta et al.2 included 499 papers, 266 of which were based on Kidney Disease: Improving Global Outcomes (KDIGO) or equivalent AKI definitions.2 The AKI incidence, according to KDIGO stages, in >4.5 million patients was 20.9%, and AKI affected 3,000–5,000 patients per 1 million of the population per year. Recent studies have described an incidence as high as 15,000 per 1 million of the population per year. Even so, AKI incidence in LMIC is still unkown and some studies have showed lower levels than in HIC. Additionally, epidemiological data from LMIC are difficult to interpret as there are heterogeneous cohorts and different methods of reporting involved, as well as huge variations in ability to diagnose and treat AKI.2
Another factor to consider is the high incidence of AKI in hospitalised patients in areas with more resources, in contrast to community-acquired AKI and patients in rural areas, where AKI is often not detected.28-31 Nonetheless, AKI in this population is often avoidable and reversible, affecting healthy and young individuals, and might be secondary to animal venoms, complications during pregnancy including septic abortion, use of herbal medicine, infectious diarrhoea, and other infectious diseases (Table 1).
ISN has collaborated with the Institute of Health Metrics and Evaluation (IHME), who have coordinated with the Global Burden of Disease Study (GBD) to include AKI in future GBD reports, which will envolve determining the relationship between AKI and disability or death. The main goal is to add strength to the concept that a high proportion of cases of AKI in LMIC are avoidable, demonstrating that investment in early recognition can reduce mortality and improve outcomes. As most studies on AKI are from developed countries and mainly focus on intensive care unit (ICU) populations, the 0by25 initiative developed two projects to assess how AKI contributes to the global burden of health loss: the AKI Global Snapshot (GSN) study, and the Pilot Study.
The GSN is a prospective observational cohort study, comparing aetiologies, risk factors, diagnoses, treatment, and AKI outcomes. The GSN was performed in 2014 and included >600 participating centres in 93 countries.10,32 Patients were classified as having community-acquired or hospital-acquired AKI and countries were classified into HIC, upper-middle-income countries (UMIC), and LMIC according to their 2014 GNI per capita, using thresholds defined by the World Bank Atlas method.33 In LMIC, 79% of AKI cases occurred in the community. Almost 50% of patients were hospitalised when AKI diagnosis occurred, with similar rates across all country categories. Hypotension and shock were the most prevalent causes in HIC and UMIC, while dehydration was the most frequent risk factor for AKI in LMIC. Most dehydration episodes were majoritively associated with inadequate oral intake (60%), followed by vomiting (44%). There was a higher number of patients with Stage 3 AKI in LMIC than in HIC and UMIC (58%, versus 47% and 41%, respectively). However, more patients in LMIC experienced recovery from AKI than patients from HIC and UMIC. The large proportion of patients presenting with Stage 3 AKI has important implications on higher mortality rate, longer hospital stay, and no recovery of kidney function 10.
In a separate analysis of children, the main risk factors for AKI in HIC were hypotension (30%), postsurgical complications (27%), and dehydration (26%). In contrast, dehydration was the most common risk factor in LMIC (43.5%) and UMIC (30.6%).34 Mortality in community-acquired AKI was higher in LMIC (11% versus 9% in HIC). In the paediatric population, this difference was higher: 3% in HIC and 20% in LMIC. In LMIC, mortality was higher among ICU patients (21%), in comparison to HIC (13%). AKI recovery was more often complete in LMIC (39%) than in HIC (33%) or UMIC (28%). Recovery rates from community- versus hospital-acquired AKI were very similar in HIC and UMIC. In LMIC, recovery occurred in 79% of patients with community-acquired AKI and in only 20% of patients with hospital-acquired AKI.
The results of the GSN underline the need to raise awareness of AKI, in order to increase the detection of patients who present with earlier stages of AKI. The study also indicates that the main causes of AKI in LMIC are dehydration, infection, and sepsis. The strategies to reduce the burden of AKI need to be based on the identification of patients at risk, implementation of preventative actions, application of diagnostic methods, and timely referral for specialist care.34,35 Development of educational and training tools for raising awareness and standardising the care of AKI cases is also essential.
The Pilot Study, not yet published, involves a prospective cohort of patients at high risk of community-acquired AKI in three different countries: Malawi, Nepal, and Bolivia.36,37 The primary aim was to evaluate the feasibility of implementing an education and training program to optimise care of AKI, based on a protocol-driven approach in rural areas. Patients were selected for signs or symptoms associated with a high risk of developing AKI. AKI was confirmed within 7 days by a serum creatinine concentration test, according to KDIGO criteria.
The results of the pilot study will provide an assessment of the diagnosis and management of AKI in community health centres and will identify barriers to optimal care of patients. It is expected to show the effect of simple interventions, such as education and provision of POCT, on the outcomes of patients with a high risk of developing AKI. The 0by25 initiative developed partnerships with the governments of participating countries to establish the best approaches to decrease avoidable deaths from AKI.
NEXT STEPS AND CONCLUSION
AKI has been associated with high mortality rates; however, it is likely that a significant number of deaths associated with AKI could be avoided. In the 0by25 initiative, the ISN has challenged both the nephrology community and the broader health care community to work collaboratively to develop effective programs to treat AKI in developing countries. Additionally, the governments of low- and middle-income countries need to be aware of the importance of sanitation, pure water supply, and basic health education, to have a chance of eradicating AKI in the foreseeable future.
The ISN 0by25 initiative has offered an opportunity to help improve education, training, care delivery, and the implementation of diagnostic and intervention studies in AKI. Additional key elements include improvement in health care and diagnostic tool availability and provision of acute RRT for those in need. The worldwide heterogeneity in the cause, setting, and progressional course of AKI demands an integrative approach.
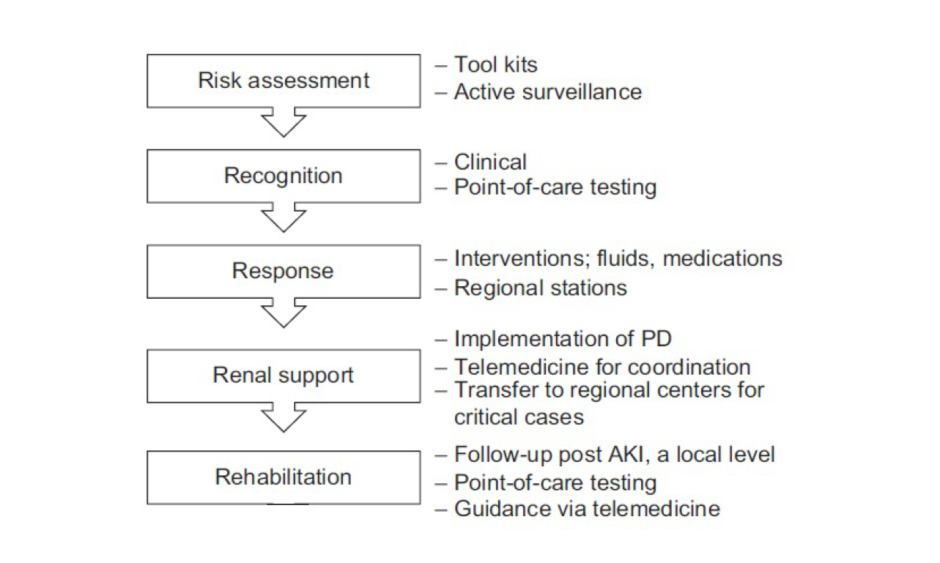
The Saving Young Lives initiative5,24 has shown that providing assistance with and promoting the development of local resources can help to generate local RRT programmes in LMIC. However, given the lack of investment and resources in LMIC, the delivery of affordable RRT to all patients with AKI in LMIC remains limited.5,22 The 0by25 initiative would like to develop a sustainable infrastructure to enable ‘need-driven’ approaches for education and training, care delivery, and measurable outcomes. This approach will be tested in future studies in selected centres in developing countries with the aim of rapidly scaling up the lessons learned for broader adoption at national and regional levels. The program was implemented according to five strategic components representing the 5R framework: Risk assessment, Recognition, Response, Renal support, and Rehabilitation approach, as detailed in Figure 1.
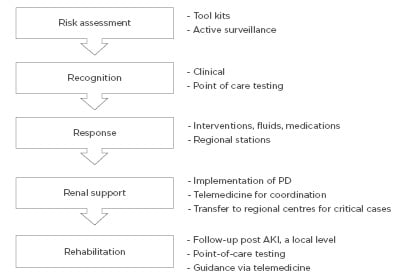
Figure 1: The 5R approach for a sustainable AKI programme by the ISN 0by25 initiative.10
AKI: acute kidney injury; ISN: International Society of Nephrology; PD: peritoneal dialysis.
The Saving Young Lives program was started in 2012 and has been providing financial and educational support to develop PD therapy for patients with AKI in developing countries.5,20-28 In these settings, the advantages of PD over HD include medical and technical simplicity and the lack of need for electricity, machinery, and pure water, among other factors.
Fluids can be a barrier to PD use for AKI treatment in LMIC. Commercially produced solutions are produced to high standards with strict aseptic technique and careful monitoring of bacterial and endotoxin contamination. Locally prepared solutions carry the potential risks of contamination and mixing errors which may be life-threatening. Commercial solutions often have closed drainage systems to prevent accidental contamination. However, the disadvantage of commercial solutions is the financial cost, which may limit utilisation in low resource settings, particularly if patients are paying for their own care. This includes both the cost of purchasing the solutions and the cost of transportation to sites providing the treatment, as well as costs such as taxes and bureaucratic assessments. However, the costs of peritonitis due to contaminated, locally produced fluid must also be considered when making decisions based on financial grounds.
The ISPD recommend the following types of fluid, in order of preference:27
- Commercially prepared solutions.
- Locally prepared fluid made in an approved and certified aseptic unit/pharmacy. These products have a limited expiry date, as approved by the manufacturing unit.
- Solutions prepared in a clean environment, with the minimum number of punctures and least number of steps. This fluid should be used immediately.
In situations where dialysis fluids are not available or are unaffordable, dialysis fluids can be prepared using available intravenous fluids. Table 2 shows some examples of intravenous fluids that can be converted into dialysis fluids. By adding glucose and/or bicarbonate to these fluids, solutions can be developed that are similar in composition to standard dialysis solutions.
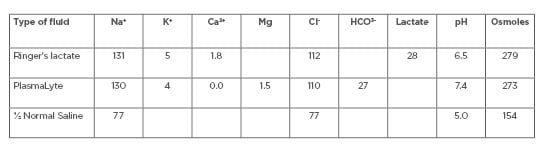
Table 2: Commercially available intravenous fluids.
Ca2+: calcium ion; Cl–: chloride ion; HCO3-: bicarbonate; K+: potassium ion; Mg: magnesium; Na+: sodium ion.
Importantly, excellent outcomes have been observed when using PD to treat patients with AKI.20-23 As a team effort, the international societies have helped to provide supplies, and education, training, and support for health care workers. To date, successful programs have been developed at 10 sites in eight countries, with three additional sites in development as of late 2015.