BACKGROUND AND AIMS
Salt restriction and hydrochlorothiazide (HCT) are known to increase the antihypertensive and antiproteinuric effects of renin-angiotensin-aldosterone-system inhibitors (RAASi) in chronic kidney disease.1-4 Additionally, potassium intake affects blood pressure (BP) and albuminuria.5-7 This effect is likely mediated through potassium’s inhibitory effect on the sodium-chloride-cotransporter, increasing natriuresis.8 However, the influence of potassium intake in response to sodium restriction and HCT during RAASi treatment in patients with diabetic nephropathy is unknown.
METHODS
To investigate this matter, the authors performed a post-hoc analysis of a randomised, double-blind, placebo-controlled cross-over trial (n=43).1 The trial investigated the separate and combined effects of low sodium (LS; aim 50 mmol Na+/d) and HCT (50 mg/d) on BP and albuminuria during standardised angiotensin-converting enzyme inhibition (ACEi; lisinopril 40 mg/d) in patients with diabetic nephropathy. Each treatment period (normal sodium [NS]; LS; NS+HCT; LS+HCT) lasted 6 weeks. The authors categorised participants as above or below the median baseline 24-hour urinary potassium excretion (UKV), stratifying by sex, as a proxy for potassium intake. BP and albuminuria outcomes were defined as a percentage reduction from baseline systolic BP (SBP) and urinary albumin-creatinine ratio (ACR). Within both subgroups, the effects of the interventions were compared to baseline using repeated measures ANOVA (SBP) or paired Wilcoxon rank-sum analysis (log-transformed ACR). Post-hoc between-group comparisons were performed with Bonferroni testing.
RESULTS
A total of 43 patients (six female; mean age 64.9±1.4 years) had baseline UKV data available and were included in this analysis. Complete ACR data was available for 33 patients. The mean estimated glomerular filtration rate was 65.2 (±3.9), mean SBP was 146.6±2.4 mmHg, and median albuminuria was 648.6 (251.2–1883.0) mg/24h. Median UKV in the high and low potassium groups was 94 (interquartile range: 90–111) and 64 (49–71) mmol/24u, respectively (p<0.0001). Baseline SBP, ACR, age, and the estimated glomerular filtration rate did not differ significantly between the groups. Although baseline sodium excretion was higher in the high potassium group (mean 191 versus 260 mmol/d; p=0.002), the percentual reduction in sodium excretion at LS was comparable between groups.
LS significantly reduced ACR in the high potassium group (p=0.011), whereas ACR reduction on LS was not significant in the low potassium group (p=0.058; Figure 1). HCT added to ACEi therapy without salt restriction reduced ACR significantly in the high potassium group (p<0.0001), but not in the low potassium group (p=0.2110). Combining LS and HCT reduced ACR in both high (p<0.0001) and low potassium (p=0.034) groups. When comparing the subgroups, the high potassium group showed a significantly larger ACR reduction (p=0.010) during HCT therapy, and a trend towards a larger reduction with HCT+LS (p=0.050). The effect of LS alone was similar between high and low potassium groups (p=0.485).
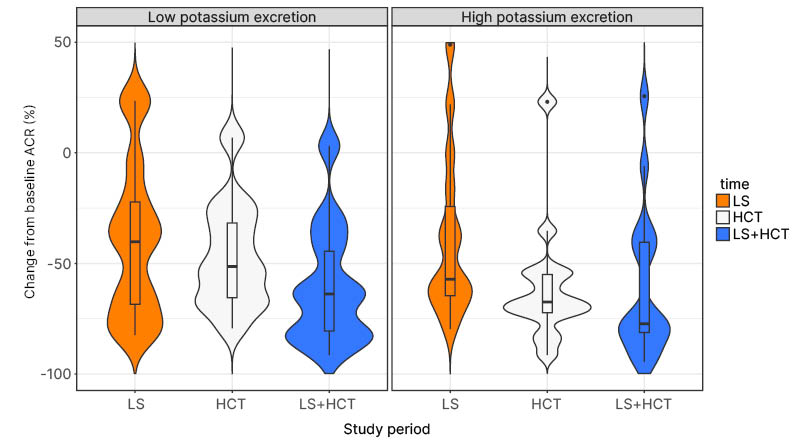
Figure 1: Violin plot showing the relative change from baseline albumin-creatinine ratio at each study period, for the low and high potassium excretion subgroups.
The high potassium group showed a significant reduction in ACR at LS (p=0.011), HCT (p<0.0001), and their combination (p<0.0001). For the low potassium group, ACR was only significantly reduced from baseline at combined LS+HCT (p=0.034).
ACR: albumin-creatinine ratio; HCT: hydrochlorothiazide; LS: low sodium.
Regarding BP, there was a significant reduction in SBP from baseline during LS in the low potassium group (p=0.003), but not in the high potassium group (p=0.208). In both groups, all further treatment conditions significantly lowered SBP from baseline. Post-hoc analysis showed no significant differences in SBP reduction between high and low potassium groups, although there was a trend towards larger SBP reduction in the low potassium group during LS (p=0.070).
CONCLUSION
The authors found that higher baseline potassium excretion was associated with stronger albuminuria-reducing effects of salt restriction and HCT in patients with diabetic nephropathy on background ACEi therapy. These effects were not mediated by similar effects on BP.







