INTRODUCTION
Drug dosing in patients with chronic kidney disease (CKD) can be challenging due to the exclusion of such patients in the clinical trials during the drug development process. It is increasingly evident that CKD can affect the elimination of drugs excreted not only by the renal route, but also through non-renal routes (bile, gut) and through metabolism (liver).1 The uremic toxins generated in CKD can affect the clearance of drugs by a multitude of mechanisms, including alteration in the function of hepatic drug metabolising enzymes.2 Citalopram is an antidepressant drug eliminated predominantly (85%) by cytochrome p450 enzyme (CYP2C19 and CYP 3A4)-mediated hepatic metabolism.
This study aimed to demonstrate the impact of CKD on the hepatic clearance of citalopram by correlating the citalopram concentration in patients with various stages of CKD and modelling hepatic clearance of citalopram with declining CKD.
METHODS
The study was conducted on 75 patients who were on regular citalopram within the Salford Kidney Study (total patient population: 3,115) between October 2002 and December 2016. One hundred and fifty citalopram levels were assayed for analysis from the available baseline and annual follow-up samples of these 75 patients by liquid chromatography-tandem mass spectrometry (LC-MS/MS). The patients were grouped into moderate (CKD stages 2 and 3) and severe CKD (CKD stages 4 and 5) based on their estimated glomerular filtration rate (eGFR). Pearson’s correlation analysis was used to correlate the citalopram concentrations with eGFR in SPSS. A single compartmental population pharmacokinetic model was generated using standard citalopram pharmacokinetic parameters and applied to compare the trend in hepatic clearance of the drug across various CKD stages using Monolix software (version 2018R1; Lixoft, Paris, France). Forty-three patients with two or more levels were used in this pharmacokinetic trend analysis.
RESULTS
The median age of the cohort was 65 years with a predominance of females (56%) and Caucasians (100%). Median eGFR of the population was 30.5 mL/minute/1.73 m2. The median dose-adjusted citalopram concentration was observed to be significantly higher in the severe CKD patient group (6.65 versus 3.78 ng/mL/g; p<0.001). In the Pearson’s correlation analysis there was a significant negative relationship between eGFR and the dose-adjusted citalopram levels (correlation coefficient [148] : -29; p<0.001) and this significance extended in the partial correlation analysis after controlling for other important confounding variables. In the population pharmacokinetic model, a 23% reduction in the mean hepatic clearance was noted in the severe CKD group compared to the moderate CKD group, which was statistically significant (p<0.001) (Figure 1). A reduction in hepatic clearance was also noted in females, patients of an older age, and those taking proton pump inhibitor tablets, a finding which is supported in previous literature.3,4
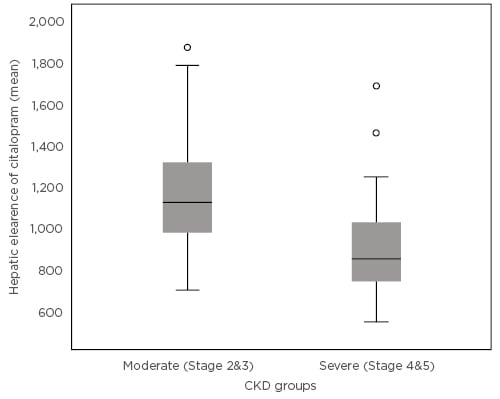
Figure 1: Mean hepatic clearance of citalopram in the moderate and severe chronic kidney disease groups.
CKD: chronic kidney disease.
CONCLUSION
The study results support the hypothesis that metabolism of drugs eliminated predominantly by the non-renal route (hepatic metabolism) can be reduced with advancing CKD, possibly due to inhibition of the hepatic drug metabolising enzyme activity by uraemic toxins. Further prospective studies are warranted to delineate these differences and aid dosing in advanced CKD. Following the presentation of these results at the European Renal Association-European Dialysis and Transplant Association 2019 Congress, discussions were focussed around the clinical implications of the project and how models can be useful in precision drug dosing in this special group population in the future.








