Abstract
Recurrent meningitis, although rare, poses a significant clinical challenge due to its potential for severe complications and profound impact on patient quality of life. This case report details a 33-year-old male with a family history of meningitis who presented with recurrent bacterial meningitis. Initial diagnostic evaluations, including CT and MRI, identified a defect in the bony cribriform plate, creating an abnormal pathway for bacterial invasion.
Despite appropriate management with intravenous antibiotics, the patient’s recurrent episodes necessitated surgical intervention. A multidisciplinary approach involving neurosurgical resection of the mass and repair of the skull base defect successfully resolved the issue, with the patient remaining asymptomatic postoperatively.
This report emphasises the importance of thorough anatomical evaluation in cases of recurrent meningitis, particularly in identifying subtle skull base defects that may not be apparent on initial imaging. The discussion reviews the relevant literature on the aetiologies of recurrent bacterial meningitis, highlighting the significant role of anatomical abnormalities and the necessity for advanced imaging techniques in diagnosis. Further research into the potential familial links and unrecognised aetiologies of recurrent meningitis is warranted to better understand and manage this complex condition.
Key Points
1. Recurrent bacterial meningitis is rare but poses significant diagnostic and treatment challenges, often requiring advanced imaging and multidisciplinary interventions to identify underlying anatomical defects.2. This is a case report and literature review describing a 33-year-old male with recurrent bacterial meningitis due to a cribriform plate defect. The authors also analyse similar cases of recurrent meningitis, growths capable of causing cribriform plate defects, and possible aetiologies of familial bacterial meningitis.
3. Familial bacterial meningitis is exceptionally rare, and recurrent cases should be thoroughly investigated for anatomical defects and genetic contributions to guide appropriate diagnosis and management.
INTRODUCTION
Recurrent meningitis is a rare but serious condition, occurring in about 5% of cases of community-acquired meningitis.1 Recurrent episodes of meningitis can result from various underlying causes, including infection, tumours, medications, and autoimmune diseases, making accurate diagnosis crucial for treatment.2 Anatomical skull defects, which can be congenital or more often acquired through trauma or mass compression, are significant risk factors for an infectious aetiology, as they create abnormal pathways for pathogens to enter the meninges.3,4
In this case report, a complex presentation of recurrent meningitis secondary to a mass lesion, leading to a cribriform plate defect and creating a pathway for bacteria to cause repeated infections, is presented. While the author’s pathologists could not definitively diagnose the specimen microscopically, the likely role of nasal polyps as the cause of the patient’s recurrent episodes is also explored. This case is particularly notable due to the patient’s significant family history of adulthood meningitis, likely indicating familial contributions, an area only scarcely analysed in the medical literature. The imaging modalities used to diagnose the condition and its potential aetiology are also discussed, in addition to the established treatment protocols and potential surgical approaches for managing skull base defects causing cerebrospinal fluid (CSF) leaks. This detailed analysis highlights the importance of comprehensive evaluation and intervention to prevent recurrent episodes and improve patient outcomes. Written consent for this report was provided by our patient.
CASE PRESENTATION
A 33-year-old male presented to the emergency department (ED) with a 3-hour history of severe generalised headache accompanied by nausea and photophobia. He also reported a sore throat, myalgias, diarrhoea, and recent exposure to a sick contact. The patient’s past medical history was significant for migraines and two prior hospitalisations for bacterial meningitis in 2017 and 2020. There was no known history of recent or remote head trauma and no prior ENT (Ear, Nose, Throat) or neurosurgical procedures. His family history included a brother and an uncle who had both passed away from meningitis. He stated he was allergic to dust mite extract, tomato, amoxicillin-clavulanate, seafood, and shellfish and was a six-pack-year smoker. Although not reported by our patient in the ED, it was later noted he also had a history of chronic rhinosinusitis.
On arrival, the patient was afebrile and haemodynamically stable; however, his condition rapidly deteriorated, leading to progressive alteration in mental status and lethargy, necessitating intubation and admission to the ICU. Laboratory investigations revealed an elevated white blood cell (WBC) count of 14.4 with neutrophilia and increased procalcitonin levels. A CT scan of the head performed on admission was unremarkable. Two lumbar punctures (LP) were done, and the findings were consistent with bacterial meningitis, as shown in Table 1. Other positive findings in the CSF are also included in Table 1. Tests for HIV, herpes simplex virus, syphilis, and West Nile virus antibodies were negative. A limited immunological workup, performed acutely secondary to the family history, was also negative, including measurement of IgG and IgM levels, complement levels, and antinuclear antibodies (ANA). An EEG demonstrated diffuse slowing, indicative of encephalopathy of metabolic-toxic origin.
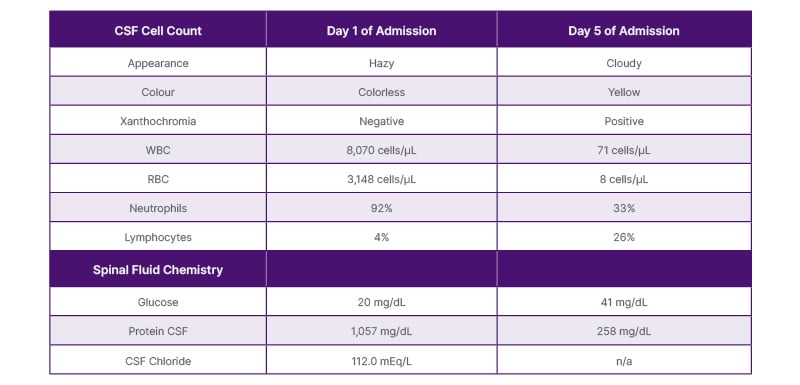
Table 1: Cerebral spinal fluid findings.
CSF: cerebrospinal fluid; RBC: red blood cells; WBC: White blood cells.
Follow-up head CT without contrast revealed mucosal-thickening in the maxillary and sphenoid sinuses, prompting further investigations. CT imaging of the sinus and maxillofacial bones, as seen in Figure 1, revealed a 4 mm defect in the bony cribriform plate and a widening of the olfactory fossa on the right. Follow-up MRI, per Figure 2, of the brain revealed an ovoid structure within the superior aspect of the right nasal cavity extending towards the cribriform plate defect measuring 0.6×0.8×0.8 cm, suggesting the presence of an intranasal glioma or dermoid cyst (Figures 1 and 2A–D).
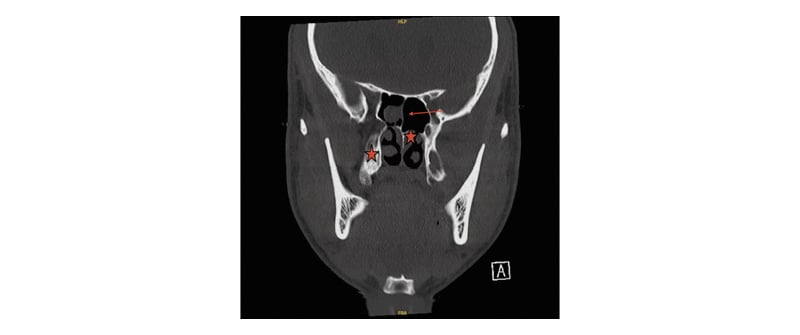
Figure 1: CT sinus maxillofacial bones without contrast.
Widening of the olfactory fossa on the right; 4 mm defect in the bony cribriform plate, marked by the arrow;
increased soft tissues medial to the inferior turbinate and superior in the anterior nasal cavity, marked by the stars.
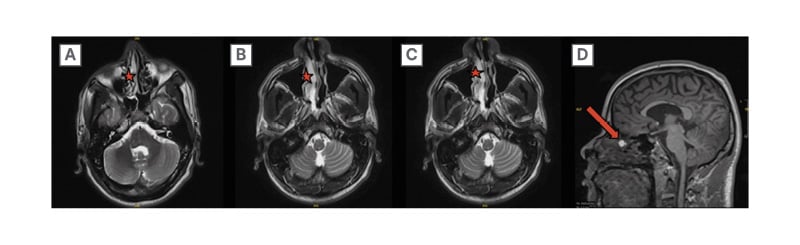
Figure 2: MRI brain with and without contrast.
Within the superior aspect of the right nasal cavity is a T1 hyperintense ovoid structure measuring 0.6 x 0.8 x 0.8 cm, marked by the stars, with corresponding lower signal on the T2/FLAIR images. On sagittal images, an arrow is marked where there appears to be a potential extending towards the cribriform plate defect seen on CT.
The patient was treated with intravenous ceftriaxone for 2 weeks and dexamethasone for 4 days. His condition gradually improved, and he was discharged on hospital Day 14, in stable condition, with a prescription for oral levofloxacin for 7 days and subsequent prophylactic antibiotics. He also received PCV 20 and PPSV 23 vaccinations.
A month after discharge, the patient underwent a nasal endoscopy to visualise the septum, turbinates, osteomeatal complexes, and sphenoethmoidal recesses. The findings of this procedure included generalised mucosal oedema with bilateral inferior turbinate hypertrophy and a deviated septum but no visualisation of any growths. The lack of clear findings prompted neurosurgical intervention to surgically resect the growth visualised on CT, causing the cribriform plate defect.
A week after the nasal endoscopy, the author’s patient went into surgery requiring both an otolaryngologist and two neurosurgeons. The otolaryngologist initiated the procedure by accessing the intranasal cavity with an endoscope. They identified the lesion at the anterior skull base, harvested a nasoseptal flap, and prepared it for later use. Using a bimanual technique, the surgeons collaboratively resected the lesion under endoscopic visualisation, noting a low-volume CSF leak from a small dural communication. The lesion and surrounding mucosa were removed and sent for biopsy, revealing thick mucus-like material, which was cultured. To repair the skull base defect, an abdominal fat graft was harvested and placed intradural and over the dural defect. A button gasket seal using alloderm and adheris was created, effectively sealing the defect. The nasoseptal flap was then secured over the closure, ensuring no further CSF egress. The procedure was completed without complications, and the patient was extubated post-operatively after placing a temporary lumbar drain for 3 days.
Outpatient MRI confirmed adequate repair of the cribiform plate defect. Approximately 1 month after discharge, the patient returned for follow-up, and he remained asymptomatic at subsequent visits, with a full recovery postoperatively. He underwent an extensive immunologic workup and was eventually cleared of his symptoms being secondary to an immunodeficiency. This was done in part secondary to the positive familial history. The anterior skull base lesion was subsequently biopsied, with the report stating only “benign sinonasal mucosa with no evidence of tumour.” This report ruled out a dermoid cyst or glioma, the leading suspicion after the MRI. However, no further comments were made about the nature of the removed pathology.
DISCUSSION
The definition of recurrent bacterial meningitis may vary depending on the source. According to a large 2022 study on bacterial meningitis, it is defined as a second or subsequent episode of bacterial meningitis, either caused by a different organism or occurring more than 3 weeks after the resolution of the previous infection.¹ In this 2022 study, 2,264 patients with bacterial meningitis were examined, and 118 (approximately 5%) met the criteria for recurrent bacterial meningitis based on this definition.¹ Thirty-two percent of these cases were secondary to CSF leakage, with the most frequent risk factor seen in patients with recurrent bacterial meningitis being parameningeal infections of the ears or sinuses at 36%.1 Immunocompromised states were involved in only 14% of cases, while 26% of subjects had no risk factors present.1 A history of otolaryngological surgery or remote head trauma were found to be the leading associations with CSF leakage, representing 27% and 24% of cases, respectively.1 These data underscore the importance of a thorough medical history and physical examination in patients with recurrent meningitis. For instance, patients may not recognise prior head trauma or chronic sinusitis as relevant to their recurrent infections.
In the authors’ case, a CSF leak was identified to be the cause of the recurrent episodes, but the tissue biopsy for the lesion around the cribiform plate defect was left without stating a definitive diagnosis. It is believed that a mass of benign sinonasal mucosa indicates a polyp, and the patient’s history of chronic rhinorrhoea supports this claim. Polyps causing recurrent bacterial meningitis are exceedingly rare and can also involve the parameningeal spread of chronic sinus infection rather than CSF leak. In a case report from South Korea, a middle-aged patient similar to the authors’ also presented to the hospital for his 3rd episode of CSF culture-confirmed Streptococcus pneumoniae bacterial meningitis.5 CT scan revealed an inflammatory polyp along the cribriform plate, confirmed explicitly by biopsy upon removal.5 This patient, however, had no signs of a skull base defect causing a CSF leak, and their episodes were deemed to be caused by parameningeal spread.5
Recurrent bacterial meningitis secondary to a polyp causing CSF leak is thought to be due to the polyp causing increasing pressure on the skull.6 Eventually, after years, significant bony erosion can occur, causing skull base defects and CSF leaks. This rare phenomenon has previously been demonstrated to be a cause of recurrent bacterial meningitis.6 It should also be noted that chronic CSF leak could instead be the source of sinonasal inflammation and subsequent polyp formation.7 Still, given the patient’s lack of trauma history and the presence of multiple allergies, the polyp was likely the initiating factor in this particular case rather than a spontaneous CSF leak.
Although the pathology report likely rules these out, other possible causes of the patient’s CSF leak include meningiomas, dermoid cysts, inverted papillomas, meningoceles, and encephaloceles. These are benign sinonasal epithelial masses capable of causing CSF leaks.8 There have been several cases of dermoid cysts causing recurrent bacterial meningitis in children, which likely contributed to the initial MRI read’s suspicion of a dermoid cyst. However, the few documented cases of these cysts presenting in adulthood have caused aseptic meningitis secondary to rupture. In the author’s review of the literature, one case of an adult patient with an encephalocele resulting in repeated episodes of bacterial meningitis was found. This patient underwent a pterional craniotomy with no known further episodes.9
Workup for recurrent bacterial meningitis should likely be initiated at the second lifetime episode. In addition to immunological tests, the above study revealing a significant amount of cases due to anatomical reasons highlights the importance of sensitive imaging studies to detect skull base defects. Although most patients will receive a non-contrast head CT before LP, it has low sensitivity in identifying skull base defects and subsequent CSF leaks. Therefore, it is recommended that further imaging be completed in cases of recurrent bacterial meningitis. As backed by the data stated above, it is also recommended that minor immunodeficiencies should not cause a complete cessation of imaging studies in the search for the aetiology of recurrent bacterial meningitis.4
Suspected CSF leaks, such as the presence of clear-coloured rhinorrhoea or otorrhoea, can also initially be investigated with a B-2 transferrin assay. This test is not diagnostic and was not performed on the authors’ patient, but positive test results will increase the urgency of precise imaging.10 The specific imaging studies should be based on the degree of clinical suspicion.
Suspicion of anterior skull base defects, as in the authors’ patient, should initially be investigated with a thin-section cranial CT focusing on the coronal cut. It has been reported that axial cuts have lower sensitivity in identifying defects in the ethmoidal or cribriform plate areas.11 Thin slice CT at 1–2 mm has also been found to be much more specific than a thicker cut of 4–5 mm.11 As seen in their patient, CT-sinuses should be performed if there is evidence of sinus pathology. The presence of CSF otorrhoea, would necessitate a CT of the temporal bone. Regardless of the type, CTs should likely be followed up with MRIs, especially when there is surgical consideration. If CSF leakage suspicion remains strong with negative CT imaging, cisternography can be performed to identify more minor defects. However, it is essential to remember that cisternography requires LP and comes with associated risks of infections. For this reason and the fact that comparison of non-contrast imaging can significantly improve interpretation quality, cisternography should rarely be the initial imaging study of choice.
Identifying the pathogen via CSF cultures is also an integral part of the diagnostic and treatment plan. Like isolated bacterial meningitis, S. pneumoniae was also found to be the leading cause of recurrent episodes, representing ~65% of cases, as seen in the authors’ patient.1 Bacteria isolation can also give clues into possible aetiologies of the episodes. For example, 92% of cases in which Neisseria meningitidis was isolated were found to also have complement deficiencies.1 In these cases, performing early terminal complement studies first, then intense imaging, could be advantageous.
Familial bacterial meningitis is exceptionally rare and therefore scantily explored. The author’s literature search yielded few explanations as to why the patient and two family members experienced meningeal infections. Immunodeficiencies are the most common cause and could, in theory, also explain the patient’s chronic rhinosinusitis. Many of these complex genetic anomalies, however, more commonly cause aseptic meningitis.12 One possibility for episodes of bacterial meningitis within a family is hereditary complement deficiency. Although these immunodeficiencies are much more often acquired, there are cases of inherited syndromes. Nevertheless, the authors’ patient underwent an extensive outpatient immunologic workup, which was nonrevealing. An immunodeficiency would also not account for the CSF leak.
Although it is unclear if this patient’s family members also had CSF leaks, inherited causes of this anatomical problem are exceedingly rare. There has been one documented case of familial spontaneous CSF leaks being secondary to underlying connective tissue disorders, which involved the formation of meningeal diverticula, a feature not present in the authors’ patient.13 Other possible causes of familial CSF leaks include cranial malformation syndromes, which can lead to encephaloceles or dermoid cysts. An inheritable meningioma syndrome such as neurofibromatosis could also result in a CSF leak due to mass effect. However, the patient’s pathology report, which describes only benign sinonasal mucosa, rules out these possibilities. The presence of a polyp could suggest an inheritable sinonasal polyposis syndrome, such as primary ciliary dyskinesia, but in the literature search, no cases of this specific syndrome were found connected to recurrent bacterial meningitis. The authors’ patient was referred to a geneticist for further evaluation into these differential diagnoses, but no documentation exists of this visit.
Treatment of recurrent bacterial meningitis in the acute stage is no different than meningitis in isolated cases. Initiating intravenous antibiotics and supportive measures promptly is crucial to avoid acute deterioration. Once the patient is stabilised, any skull base defects should be addressed surgically. Endoscopic techniques are the preferred approach for managing these defects.14
There are several surgical techniques for repairing cribriform CSF leaks, including the overlay technique, where a free mucosal graft from the opposite septum is placed over the defect and stabilised with SurgiCel (Ethicon Inc., Raritan, New Jersey, USA). Other approaches include the interlay, underlay, and sandwich techniques, which position the graft between, beneath, or in multiple layers of tissue, respectively. The bath plug technique uses a fat graft to fill larger defects, often followed by a mucosal graft for support. The gasket seal technique, used on this patient, involves layering grafts to seal the defect tightly. Additionally, abdominal fat, bone, temporalis fascia, nasal septum mucosa, and conchal cartilage have been used as graft materials.15 While these techniques vary in complexity and donor site morbidity, the use of an underlay or multilayer graft, as employed in this patient, is generally reserved for larger and deeper defects. The choice of graft material is often determined by the size of the defect, with larger defects requiring stronger materials, such as bone or muscle.14,16 However, the type of graft used has not been shown to significantly affect the overall success rate.17
Now nearly 1-year post-op, the patient has had no further episodes of meningitis. He has followed up with both ENT and immunology specialists without complications. He has also had no documented rhinorrhoea since the surgery. These findings support that this patient’s CSF leak was indeed patched. However, it is essential to consider that this patient’s prior episodes of meningitis occurred multiple years apart. Close follow-up to ensure the source of his infections was rectified will be essential for multiple years. This is particularly important when considering that up to 41% of patients will have an additional episode post-surgical repair.1 In these cases, further investigation into alternative causes should be conducted.
CONCLUSION
Bacterial meningitis is a severe and life-threatening condition that requires quick and effective treatment to prevent serious sequelae. Structural skull abnormalities, chronic infections of adjacent sites, or immunodeficiencies may allow for the reintroduction of bacteria, leading to recurrent infections. While immunodeficiencies should be evaluated in cases of recurrent bacterial meningitis, anatomical defects are a more common underlying aetiology. These defects, often affecting the skull base or cribriform plate, create a channel for bacteria to gain meningeal access. While these anatomical deformities may be present at birth, they also appear secondary to trauma or through bony erosion from tumours, cysts, and, in the authors’ patient, a likely polyp. Due to the significant prevalence of structural abnormalities in this patient population, it is crucial to obtain a thorough trauma history and proper imaging when investigating the underlying cause. Understanding the aetiology of the infections is necessary to guide treatment and prevent future occurrences. Secondary to the scarceness of recurrent meningitis episodes, research is still needed to gain more precise data on the frequencies of these aetiologies, particularly of such involving CSF leaks.
This is of particular importance when considering the possibility of recurrent familial meningitis. This case is unique because familial bacterial meningitis secondary to polyposis has not previously been explored before in the literature. The authors’ patient highlights the potential and unresearched role of familial inheritance in bacterial meningitis.







