Abstract
Melioidosis is an infection caused by the gram-negative bacteria Burkholderia pseudomallei. The infection is endemic in South Asia and Australia, and several risk factors have been described for acquiring the infection, the most prominent among them being diabetes. Active malignancy is not a recognized common predisposing condition for this infection, but there have been several case reports of patients with underlying malignancies who have been diagnosed with concomitant melioidosis. But the increasing use of corticosteroids along with chemotherapy-induced immunosuppression could be factors that could lead to a possible rise of the infection in this patient population. The recognition of the infection is challenging due to nonspecific clinical features, but arriving at the diagnosis is crucial in view of the protracted course of antibiotics needed to treat the acute infection, while also giving eradication therapy to prevent recurrences. The authors describe a series of three cases of melioidosis in patients with active malignancy, each highlighting a different aspect of treatment of the infection.
Key Points
1. This article describes a series of cases of infection with Burkholderia pseudomallei in patients who had an underlying malignancy. While melioidosis is an infection that is endemic to South Asia and Australia, its incidence is underreported in several countries where it is endemic.
2. Melioidosis has not historically been associated with malignancies, but with the concomitant use of corticosteroids increasing, melioidosis could be a potential infection in this population of patients.
3. The diagnosis of melioidosis is challenging, and treatment requires a protracted course of antibiotic therapy, which could potentially add to the cost of cancer care, and inadvertent delays in the continuation of antineoplastic therapy.
INTRODUCTION
Melioidosis is an infection encountered in tropical climates, endemic to South Asia and Northern Australia. Burkholderia pseudomallei, a gram-negative bacillus found in soil and water, is the causative organism. Transcutaneous inoculation, inhalation, or ingestion have all been described as modes of transmission.1 Person-to-person mode of transmission is rare, and transmission of melioidosis via sexual contact remains unproven.2 Several risk factors have been described for the occurrence of infection, prominent among them being diabetes. Chronic renal disease, alcohol use, thalassemia, and occupational exposure have all been described as risk factors in literature.3,4 There have been sparse reports of the infection occurring in patients with active malignancy and receiving chemotherapy. This case series describes the authors’ experience in treating three cases of melioidosis in patients with active malignancies, all of whom were residents of southern India.
CASE REPORTS
Case 1
A 76-year-old male, who was on second-line therapy for relapsed multiple myeloma with lenalidomide, presented with a history of fever, decreased appetite, and bilateral lower limb edema. There was a history of intermittent fever spikes over a 2-week period. The patient was evaluated after admission. On initial examination, the patient was drowsy but arousable. There were no focal neurological deficits, and respiratory examination did not reveal any significant findings. A chest X-ray was performed for source identification, which showed lung infiltrates (Figure 1). Initial blood investigations revealed an elevated C reactive protein level (134.6 mg/L), and a complete blood count showed a total leucocyte count of 11.6×109 /L with a neutrophilic predominance. Empirical antibiotics were started (cefoperazone + sulbactam). Blood cultures drawn on the day of admission were reported on Day 5 as positive for Burkholderia pseudomallei. Antibiotics were changed as per sensitivity pattern, and he received 14 days of intravenous (IV) meropenem. The patient improved symptomatically with defervescence of fever spikes and improvement in sensorium. Three days after discharge, the patient presented with worsening fever spikes and altered sensorium. Repeat blood cultures showed further growth of Burkholderia pseudomallei. The patient was started on parenteral antibiotic therapy with IV ceftazidime, which was again administered for a period of 14 days. The patient was counseled regarding the need for long-term oral antibiotic therapy and started on eradication therapy with oral cotrimoxazole for 3 months. The patient completed the course of eradication therapy, and while on the same he was restarted on anti-myeloma therapy with a rechallenge of the combination of cyclophosphamide, bortezomib, and dexona. The patient is currently on follow-up receiving maintenance bortezomib.
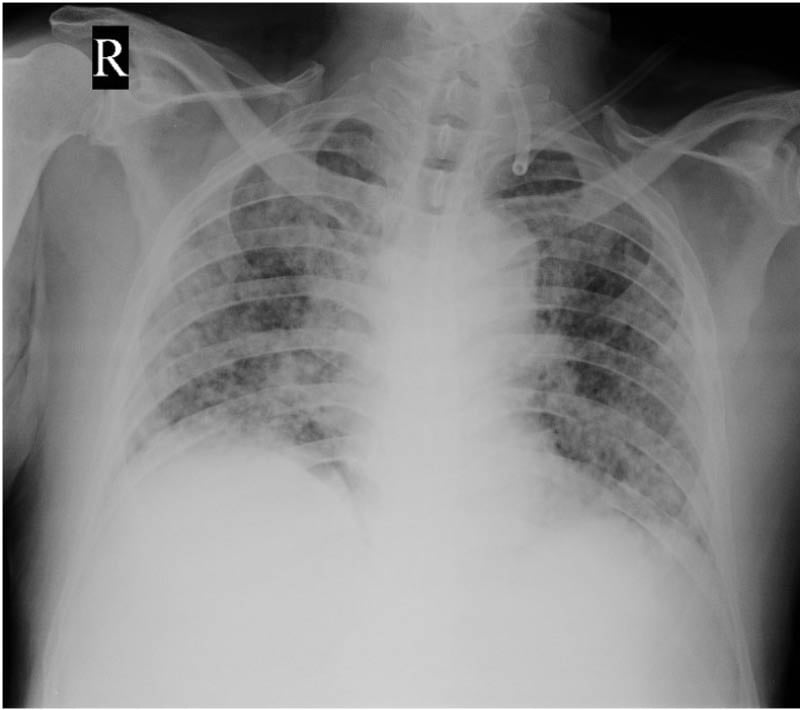
Figure 1: Initial chest X-ray in Case 1 showing lung infiltrates.
Case 2
A 54-year-old female patient, who had been diagnosed with multiple myeloma and was on first-line treatment with a bortezomib-based regimen, presented with a history of progressively worsening dyspnea. It was also known that the patient had Type 2 diabetes. Later it was also found that the patient had hyperglycemia, which was initially managed with intravenous insulin and later switched to subcutaneous basal bolus insulin. The patient underwent a high-resolution CT thorax because of persistent dyspnea, which showed lobar consolidation in the right lower lobe with associated minimal pleural effusion (Figure 2). Because of reduced IgG levels, suggestive of immune paresis, the patient received a dose of intravenous immunoglobulin. Empirical antibiotic therapy with IV cefaperazone + sulbactam was started and continued till Day 4 of admission, when blood cultures were found to be positive for Burkholderia pseudomallei. The patient was started on ceftazidime injection and received the same for a duration of 3 weeks, followed by eradication therapy with cotrimoxazole, for a total of 3 months. The patient continued antimyeloma treatment with bortezomib, lenalidomide, and dexona. One month after the initial acute episode, the patient developed a persistent cough with dyspnea on exertion; imaging showed a recurrent right-sided pleural effusion, which was drained, and cultures were sterile. The patient underwent a successful autologous stem cell transplantation after the completion of eradication therapy and obtaining clearance from the pulmonologist in view of a mildly restrictive pattern on pulmonary function tests, and is currently on regular follow-up.
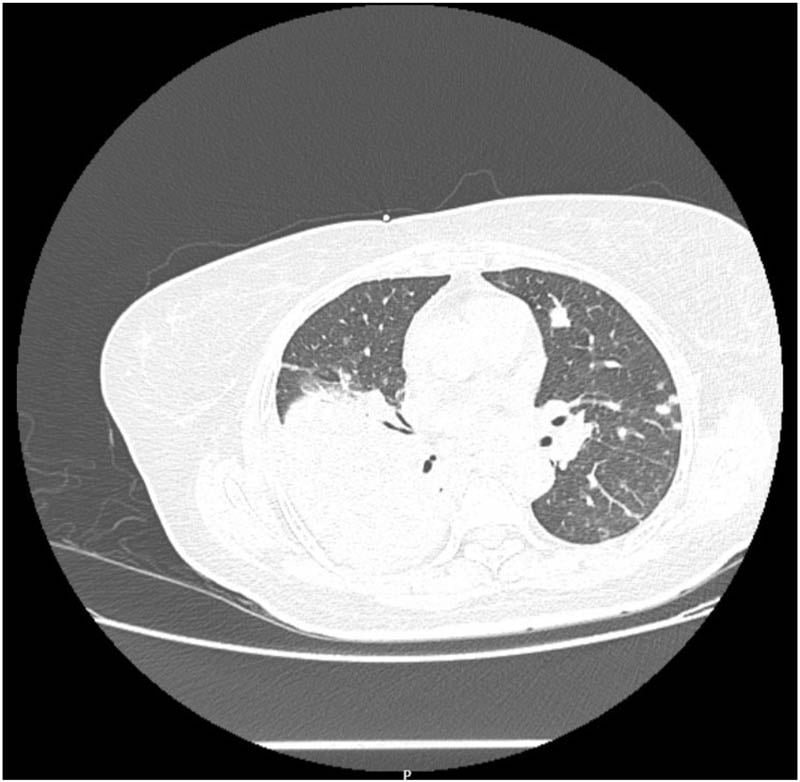
Figure 2: CT Thorax of Case 2 showing right lower lobe consolidation with associated pleural effusion. associated scattered soft tissue densities in bilateral lung fields.
Case 3
A 33-year-old patient, a diagnosed case of metastatic pancreatic neuroendocrine carcinoma with liver metastases, who was receiving chemotherapy with the combination of cisplatin and etoposide, presented with a high-grade fever for 2 days. The patient was febrile on examination (104 °F), but clinical examination did not reveal any focus of infection. Broad-spectrum antibiotics (cefaperazone + sulbactam) and other supportive measures were started. Blood cultures drawn on the day of admission were positive for Burkholderia pseudomallei. Antibiotics were changed to IV ceftazidime, in consultation with the infectious disease specialist. Ceftazidime was given at a dose of 2 gm IV every 8 hours for a duration of 14 days, the same was administered on an outpatient basis from the day care facility. Eradication therapy with trimethoprim-sulfamethoxazole was started, but in view of poor tolerance to the drug, eradication therapy was changed to oral levofloxacin at a dose of 750 mg for a duration of 3 months. The patient continued his scheduled chemotherapy, after a delay of 1 week during the acute febrile period of the infection.
DISCUSSION
Melioidosis is an infectious agent that is endemic to tropical climates, with South Asia predicted to bear 44% of all cases.1 There have been suggestions that the incidence of melioidosis has been underreported in approximately 45 countries where it is considered endemic. The past decade has seen a sharp rise in the reporting of cases from several south Asian and southeast Asian countries, including India.1,5 The diagnosis of the infection remains challenging due to several factors, the signs and symptoms of the disease are nonspecific, and there is considerable overlap with other diseases endemic to these regions, such as tuberculosis. Melioidosis can affect any organ in the body, and clinical manifestations can include pneumonia, skin, and soft tissue infections. Neurological infections, abscess formation, and septic arthritis are also known manifestations of the infection. The occurrence of fulminant sepsis without an obvious focus of infection has also been reported. Pneumonia is the most common manifestation, and the majority of patients are bacteremic. The presence of bacteremia is associated with a more fulminant clinical course.2,6 Several risk factors have been attributed to the development of the infection. In a case-control study, Suputtamongkol et al.7 tried to identify the risk factors associated with acquiring the infection. Diabetes, renal impairment, and occupational exposure to contaminated soil and water were all demonstrated to be significant risk factors. Diabetes, in addition, was the sole factor found to have a significant association with the occurrence of bacteremic melioidosis. In this study, 60.9% of the patients had underlying diabetes as a risk factor, while only 4.2% had underlying hematological malignancies or solid tumors.7
There have only been a few reported cases of melioidosis in patients with underlying malignancies (Table 1). McIntosh et al.8 reported the case of a patient with non-small cell lung cancer receiving cytotoxic chemotherapy who developed melioidosis but completed the planned chemotherapy without interruption.8 Sukauichai et al.4 reported on a patient with Stage III carcinoma of the breast receiving neoadjuvant chemotherapy who developed neutropenic sepsis secondary to melioidosis bacteremia. Two cases were reported by Mukhopadhyay et al.11 on fatal pulmonary melioidosis in patients with febrile neutropenia following chemotherapy; both patients were non-diabetic.4,11 Melioidosis has also been reported to be mistaken for a diagnosis of malignancy. Baker et al.12 reported on a series of seven cases of melioidosis, which were initially presumed to be malignancies based on imaging and clinical findings. One of these cases was a patient with known lymphoma, and the infectious process was initially suspected to be a progressive disease.12 In larger epidemiological studies, there have been considerable variations in the reported incidence of melioidosis in patients with underlying malignancies. Suputtamongkol et al.7 suggested that patients with hematological and/or solid malignancies were not more likely to develop melioidosis, and the immune impairment secondary to neutropenia did not possibly contribute to any enhanced risk for melioidosis. Currie et al.13 showed malignancy to be an underlying risk factor in 6% of their patients but led to mortality in 26% of those patients, yet this was not statistically significant.7,13
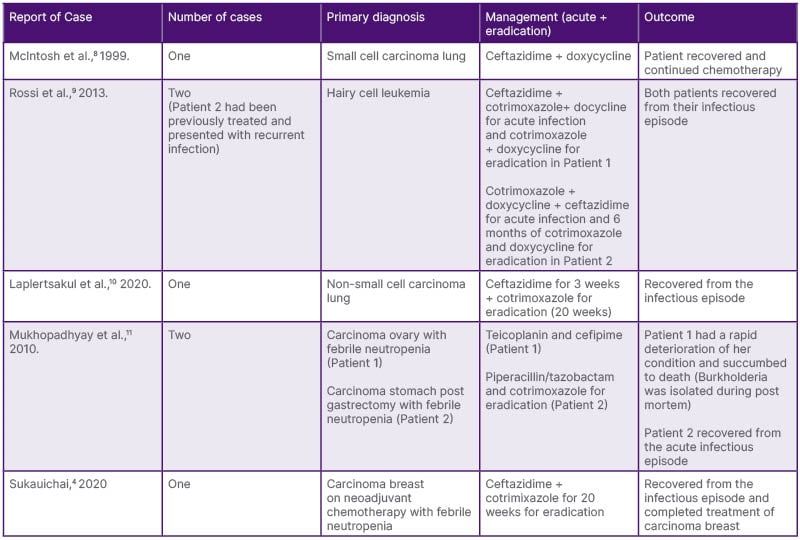
Table 1: Previously reported cases of melioidosis in patients with active malignancy.
All three of the patients described here had a diagnosis of active malignancy, and only one patient had a history of underlying diabetes, while none of the three patients had a history of febrile neutropenia. All patients had received corticosteroids as part of the management of the malignancy, which has been postulated to increase the severity of the infection due to its immunosuppressive effects.14 Impairment of host immunity has been found to play a major role in the pathogenesis of the disease. Immune suppression secondary to the use of corticosteroids and cytotoxic chemotherapy are factors that mediate the risk of melioidosis in patients with active malignancies. Studies looking at the clinical outcomes following discharge from the hospital have been limited, with limited evidence showing that a quarter of patients required re-admissions, and a quarter of these patients required multiple re-admissions.11,14 In this case series, Patient 2 had to be readmitted as described, and residual restriction in pulmonary function tests was a concern while the patient was taken up for autologous stem cell transplantation.
The treatment of melioidosis consists of two phases, of which the acute phase is aimed at preventing mortality with high dose intravenous antibiotics. A minimum 14-day course is currently recommended, with the use of either a cephalosporin (ceftazidime) or a carbapenem (meropenem). Carbapenem is often reserved for the more critically ill patients.15 Eradication therapy is aimed at eliminating any residual lesion that might relapse and is recommended for an extended duration. Relapses have been recorded in 13–23% of patients from Thailand and up to 6% of patients from the Darwin study in Australia, but with the initiation of eradication therapy recurrences have now become rarer.16 Oral eradication therapy with cotrimoxazole should be given for a minimum of 12 weeks, which can be further extended to 24 weeks.17 Other approaches for eradication therapy have been studied, combination therapy with doxycycline did not have any additional benefit compared to cotrimoxazole alone. Levofloxacin is another option but is not generally recommended due to its high degree of resistance. Rifampicin has also demonstrated strong bactericidal activity but is associated with a high degree of resistance.18
There was a significant delay in the commencement of definitive therapy for malignancy in Case 1, while poor initial compliance with eradication therapy led to rapid recurrence of the infection in the same patient. All three patients had prolonged hospitalization, with a median duration of approximately 15 days. Nevertheless, all three patients recovered from the infectious episode and were able to continue with further lines of management for their underlying malignancy (Table 2).
Melioidosis is a relatively uncommon cause of infection in patients with cancer, but the increasing use of corticosteroids and the immunosuppression associated with chemotherapy can predispose patients with active malignancies to the infection. The requirement of a protracted course of antibiotic therapy makes its recognition crucial, as in the absence of adequate eradication therapy there is a significant risk of recurrence. The residual sequelae of the disease can also complicate further treatment of the underlying malignancy, as in Case 2, where there was an added risk for autologous stem cell transplantation because of a mild restriction in lung function.
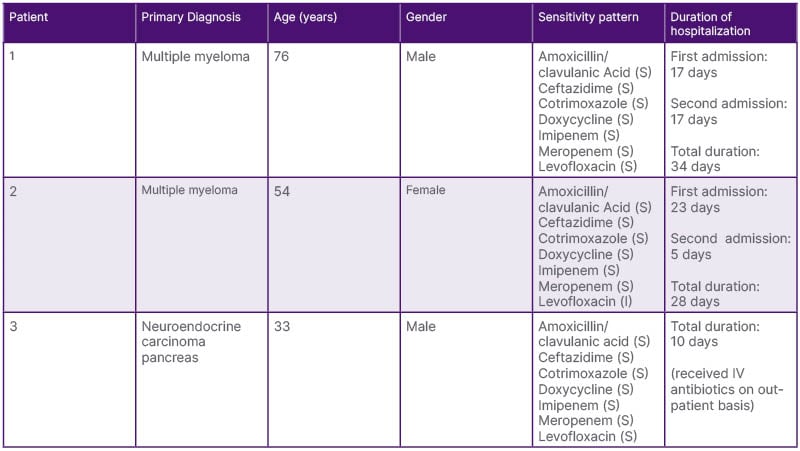
Table 2: Antibiotic sensitivity pattern and duration of hospital stay due to melioidosis.
CONCLUSION
Melioidosis is an uncommon infection but is underreported in regions where it is endemic (South Asia, Australia). The association of malignancy with the risk of acquiring melioidosis has not been as strong as that with other risk factors like diabetes, as shown in several studies. The increased use of corticosteroids in combination with cytotoxic chemotherapy has been suggested as a factor that could increase the incidence of melioidosis in patients with active malignancies in several studies. Of the three patients described here, all had an underlying diagnosis of active malignancy, and only one patient had previously been diagnosed with diabetes. All patients had received extended courses of antibiotics and prolonged hospitalization, including re-admission in one of the patients, leading to delays in commencing definitive treatment of the underlying malignancies. Thus, in the patients described, melioidosis added significantly to the burden of cancer care.







