Abstract
The coronavirus disease (COVID-19) outbreak has led to swift efforts to learn about its clinical course, prognostic markers, and complications. Consequently, there is a lot of scattered information available regarding severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) but its pathophysiology is still poorly understood. Gross and microscopic findings are very important for understanding any disease, including COVID-19. This literature review examines and summarises the biopsy, gross autopsy, and other histopathological findings that have been reported in various organs in COVID-19 patients to increase the understanding of the disease. Many histopathological findings in various organs were nonspecific, especially in the liver and brain, while others were particular to SARS-CoV-2. Therefore, further histopathological studies and autopsies are necessary to obtain consistent and reliable findings in those with COVID-19 to fully understand the pathogenesis of the disease and the impact it has on individual organs.
INTRODUCTION
The analysis of biological samples has always played a vital part in understanding pathological processes of diseases. As the outbreak of the coronavirus disease (COVID-19) turned into a global pandemic, new information has emerged regarding various aspects of the disease. However, the current understanding about the precise nature and reaction patterns in various organs and tissues in response to this infection is lacking and poorly understood. In the large number of original studies available on the subject matter, it can be difficult to get a full picture of the effect that COVID-19 has on the body as a whole, as many studies show conflicting results. In this literature review, the authors examined and summarised the macro- and micropathological findings present in each organ system in a COVID-19 patient in already published literature.
METHODS
Until 11th July 2020, PubMed and Embase were searched for related published articles. In both electronic databases, the following search strategy was applied and these keywords (in the title or abstract) were used: “COVID 19” OR “coronavirus” AND “biopsy” OR “autopsy” OR “histopathology”.
RESULTS
Lungs (n=215 cases)
A reported gross examination was available for 89 cases that tested positive for COVID-19. The macroscopic examination in all cases showed findings that ranged which patchy to diffuse areas of consolidation to severe and extensive suppurative infiltrates. A common finding was an increased lung weight in 70 cases;1-9 they were more than one standard deviation heavier than average weight. Lungs consisting of firm parenchyma with regions of dark-coloured to reddish haemorrhagic areas, with focal demarcation and severe congestion were found in 38 cases.3-5,7-12 Visible pulmonary thromboembolism were noted in 27 cases,3,13-16 some of them with associated infarcts after prophylactic dose anticoagulant therapy, suggesting that pulmonary thrombi were formed despite anticoagulant therapy.13 In some cases, visibly enlarged peritracheal and peribroncheal lymph nodes were found.7,10,11
Histopathological examination was available for 190 COVID-19 positive cases. The most commonly reported histopathological finding was diffuse alveolar damage, reported in all but one case17 ranging from hyaline membrane formation, alveolar wall oedema, fibrin deposits within the alveoli, severe capillary congestion and severe syncytial cell and Type II pneumocyte hyperplasia, and desquamation to diffuse necrosis of alveolar lining cells.2,4-6,8-13,15,18-27 One recently performed prospective cohort study reported that diffuse alveolar damage was the primary abnormality in hospitalised patients and almost every patient positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) died without medical intervention.1 Predominantly, inflammatory CD3+ and CD4+ T cells were found near precapillary and postcapillary vessel walls along with macrophages and abundant polymorphonuclear leukocytes whereas the number of CD8+ T cells was lower in comparison in most cases.2,5,6,10,16,17,19,28,29 CD61+ megakaryocytes were abundantly found actively producing platelets and located within pulmonary capillaries, further indicating the stimulation of the coagulation cascade.6,5,20
Another broad finding was pulmonary thromboembolism with fibrinous thrombi in small and large pulmonary arterioles, in some cases with haemorrhagic foci.5,6,20-23,30 In a comparative study, the lungs presented structurally deformed capillaries, showing abrupt calibre changes such as intussusceptive pillars within the capillaries and hallmarks of endothelial cell swelling, loss of contact with basal membrane, and disruption of intercellular junctions on electron microscopy.2 In multiple cases, ultrastructural examination showed distended cytoplasmic vacuoles within the pneumocytes with viral cytopathic changes and intracellular SARS-CoV-2. Virions were seen extracellularly among the cilia and within the cytoplasm of respiratory epithelial cells in the upper airway.2,6,7,10,14,18,22,27 Immunohistochemistry of viral antigens was positive in the upper airway, bronchiolar and submucosal gland epithelium, Type I and II pneumocytes, alveolar macrophages, and lung hyaline membranes.22 Superimposed bronchopneumonia was described in 19 cases with mostly neutrophilic infiltrate.4,19,20
Cardiovascular system (n=67 cases)
Cardiovascular gross findings were reported in 19 autopsy cases that tested positive for COVID-19. The most commonly reported gross findings in seven cases were large vessel occlusions characterised by intimal and medial thickening with luminal narrowing.31-33 Other reported gross findings in two cases included thin myocardial trabecula, streaking of right atrial wall myocardial tissue,7 and mottling parenchyma with a soft and rubbery texture.6 The most significant gross findings generally seen were cardiomegaly and right ventricular dilatation, which were attributed to pre-existing underlying diseases such as hypertension, diabetes, and obesity. No significant gross findings were observed in the remaining 10 cases.
Histopathological autopsies findings were available for 59 cases of COVID-19 positive patients. Unlike in other similar viral infections that caused viral myocarditis, there were no reported cases of COVID-19 myocarditis. The most commonly reported histopathological finding was myocyte necrosis, observed in almost all the cases except for one case.7 Changes to the myocardium included irregularity in shape with a darkened cytoplasm, though these changes were not sufficient for interpretation as acute myocardial injury in four cases;24 mild myxoid oedema; mild myocyte hypertrophy; focal nuclear pyknosis, in one case;7 and moderately enlarged cardiomyocyte with hyperchromatic nuclei and vacuolar degenerative change in three cases.31 The presence of diffuse lymphocytic endothelitis and apoptotic bodies were shown in 34 cases.32-35
A study proposed that SARS-CoV-2 might not directly impair the heart, because they observed scarce interstitial mononuclear inflammatory infiltrates in the myocardial tissue without substantial cardiac muscle tissue damage in their patient with COVID-19.36 There was also evidence of direct viral infection of endothelial cells, endothelitis with diffuse endothelial inflammation, and micro- and macrovascular thrombosis, both in the venous and arterial circulations, in 26 cases.34
In conclusion, cardiomegaly was most likely a result of pre-existing heart disease. Although histopathological cardiac findings were notable for absence of lymphocytic myocarditis, myocyte necrosis was the most commonly reported finding.
Gastrointestinal (n=24)
The most common gross finding was multiple and diffuse punctate haemorrhages in the stomach (n=3) and duodenal mucosa (n=1).37,38 Other gross findings (n=2) included increased liver volume and an enlarged gallbladder.37,38
In all of the 24 cases, there was mild-to-moderate hepatic lobular or portal lymphocytic infiltration.18,19,24,38,39 Four cases showed centrilobular sinusoidal dilatation.24 Minimal-to-mild micro- and macrovesicular steatosis was seen in ten cases.18,19,38 Patchy and multifocal hepatic necrosis were seen in 13 cases.19,24,38 These findings were mostly nonspecific and can be attributed to direct viral-induced cellular injuries and potential hepatotoxicity from therapeutic drugs, or be representative of pre-existing chronic liver disease that was exacerbated during COVID-19, and COVID-19-related hyperinflammatory reactions.40 SARS-CoV-2 particles without membrane-bound vesicles in hepatocyte cytoplasm were also identified.38
Central nervous system (n=79 cases)
Clearly reported gross examination was available for 41 cases that tested positive for COVID-19. The macroscopic examination showed findings that ranged from mild swelling and cerebral oedema in three cases,4,41,42 to disseminated haemorrhagic lesions throughout the cerebral white matter (size ranging from 1 mm to 1 cm) and scattered punctate subarachnoid hemorrhages in two cases.41,43 Hydrocephalus internus was present in two cases.4 No abnormalities were seen in 23 cases apart from those which were already associated with the patients’ prior comorbidities such as atherosclerosis and Parkinson’s disease (n=16).4
Histopathological examination was available for 65 COVID-19 positive cases. Here, 33 cases showed no microscopic abnormalities; one case mimicked glioma on imaging but on histopathological examination was negative for malignancy and positive for encephalitis.44 Reactive gliosis, neuronal satellitosis, perivascular haemorrhage,45 and disseminated haemorrhagic cerebral white matter lesions41 were found in eight, five, one, and one case, respectively. Other neuropathological findings included characteristics resembling vascular and demyelinating lesions. For example, the scattered necrotic neurons of acute hypoxic injury with red degeneration and oedema in the cerebrum (cortex), cerebellum (Purkinje cell layer), and hippocampus (CA1) were observed in 19 cases.41,46 Infarcts in brainstem, deep grey nuclei, or spinal cord were absent. In one case, the subcortical white matter had macrophage clusters with axonal injury and a perivascular acute disseminated encephalomyelitis-like appearance.41 No thrombi, vasculitis, or abnormalities of the olfactory bulb were observed in any cases.39,41 Focal leptomeningeal inflammation was detected in one brain specimen46 while no inflammation was present in the leptomeninges of another case.41 Perivascular lymphocytic clusters were detected in three brain specimens.41,46
Kidney (n= 43)
The histopathological effects of COVID-19 in kidney tissues were reviewed for 43 cases. Here, 32 cases reported severe acute tubular necrosis, showing diffuse proximal tubular injury with the loss of brush border, vacuolar degeneration, mild fibrosis in the interstitium, and lymphocyte infiltration on microscopy while severe glomerular injury was absent.47-49 The vacuoles seen on light microscopy correlated with double-membrane vesicles containing virion particles by electron microscopy.49 Infrequent haemosiderin granules, pigmented casts, and lumen-obstructing red blood cell aggregates were also observed. Vasculitis, interstitial inflammation, or haemorrhage were absent.48 Electron microscopy demonstrated virus-like particles are in the tubular epithelium and podocytes.48 The virus was not only directly cytotoxic, but also had the ability to initiate CD68+ macrophage and complement C5b-9 deposition to mediate tubular pathogenesis.47,50
In a separate study that evaluated 10 patients with proteinuria and COVID-19, all renal biopsies showed renal tubular acidosis without evidence of the virus in the biopsied tissue.51
Skin (n=111)
The clinical presentation of skin in COVID-19 patients showed findings including urticarial, vesicular, and petechial or purpuric eruptions; erythema multiforme; vascular complications such as acro-ischaemia, livedo, necrosis, or gangrene and chilblain; and maculopapular eruptions including morbilliform rash, plaques, and pityriasis rosea.52
Histopathological examination was available for 30 COVID-19 positive cases. The most common finding, present in 18 cases, was significant degree of perivascular infiltration of the superficial dermis by lymphocytes, eosinophils, and neutrophils in vasculitic pattern with accompanying papillary oedema and extravasation of red blood cells.44,53-58 Thrombogenic vasculopathy, found in seven cases, was associated with widespread epidermal or papillary dermal or dermohypodermal and adnexal necrosis. Adnexal necrosis spared ducts, but was present in the secretory portion of sweat coil.54,56,58 Other epidermal findings included spongiosis, basal cell vacuolation, suprabasal acantholytic ballooning keratinocytes, parakeratosis, and dyskeratotic.56,57 Superficial and deep dermal lichenoid infiltrate was also found in one case of chilblains.53 Immunohistochemistry showed extensive C5b-9 deposition within the vessels of the dermis.54
Testes (n=13)
Histopathological examination was available for 13 COVID-19 positive cases. Out of these, 11 cases showed swollen, vacuolated Sertoli cells. Intratubular cell detachment from basement membranes of the seminiferous tubules was noted. No inflammatory cells were found within the seminiferous tubules while the interstitium showed oedema and infiltration of T lymphocytes and histiocytes. Out of the same 11 cases, 18.2%, 45.5%, and 36.4% of cases showed <10%, 10–50%, and >50% injury to seminiferous tubules, respectively, while mild to no tubular injury was present in five cases of COVID-19 negative controls. The Leydig cell count was also significantly lower than in the control group. Normal spermatogenesis was observed in all cases as ACE-2 was not expressed in spermatogonia but widely present in Sertoli and Leydig cells.59 The rest of the two cases showed orchitis.45
Table 1, 2 and 3 show the patient demographics such as sex, age, comorbidities, and country the study was conducted in.
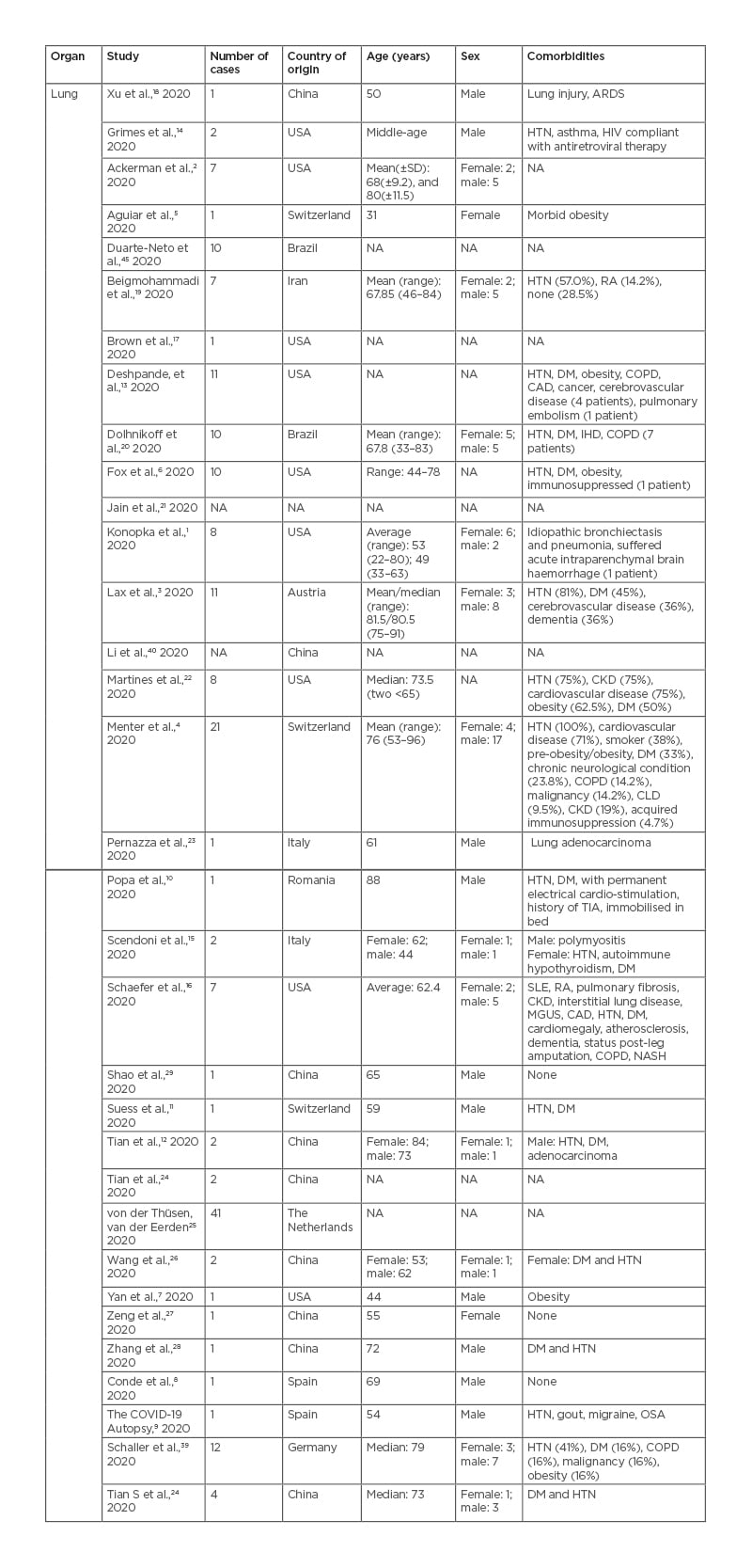
Table 1: Pulmonary findings in patients with coronavirus disease (COVID-19).
ARDS: acute respiratory distress syndrome; CAD: coronary artery disease; CKD: chronic kidney disease; CLD: chronic liver disease; COPD: chronic obstructive pulmonary disease; DM: diabetes mellitus; HTN: hypertension; IHD: ischaemic heart disease; MGUS: monoclonal gammopathy of undetermined significance; NA: not available; NASH: non-alcoholic steatohepatitis; OSA: obstructive sleep apnoea; RA: rheumatoid arthritis; SLE: systemic lupus erythematosus; TIA: transient ischaemic attack.
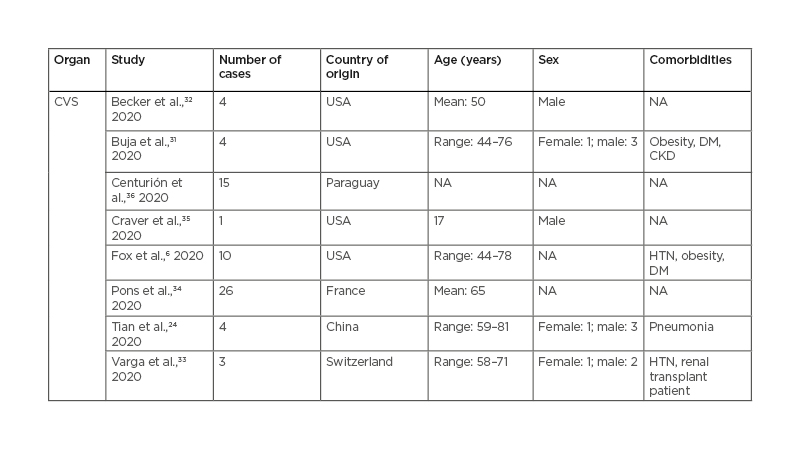
Table 2: Cardiovascular findings in patients with coronavirus disease (COVID-19).
CKD: chronic kidney disease; CVS: cardiovascular system; DM: diabetes mellitus; HTN: hypertension; NA: not available.
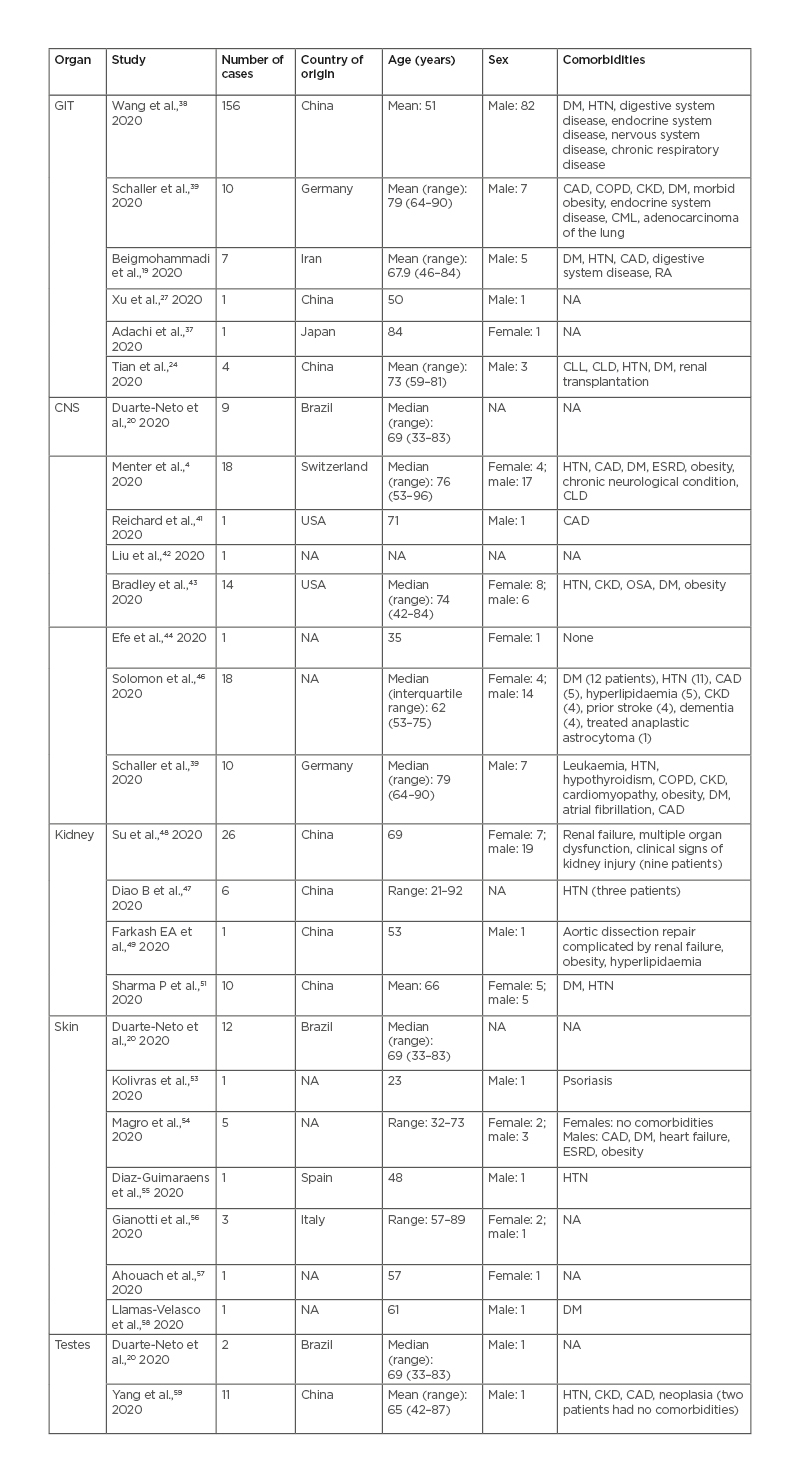
Table 3: Findings in other organ systems in patients with coronavirus disease (COVID-19) (gastrointestinal tract, central nervous system, renal, skin, testes).
CAD: coronary artery disease; CKD: chronic kidney disease; CLD: chronic liver disease; CLL: chronic lymphocytic leukaemia; CML: chronic myelogenous leukaemia; CNS: central nervous system; COPD: chronic obstructive pulmonary disease; DM: diabetes mellitus; ESRD: end-stage renal disease; GIT: gastrointestinal tract; HTN: hypertension; NA: not available; OSA: obstructive sleep apnea; RA: rheumatoid arthritis.
LIMITATIONS AND RECOMMENDATIONS
The data or cases available for review were very limited as many hospitals discontinued autopsies on COVID-19 cases to limit the spread of the virus. Further, larger studies are needed to assess the effect of COVID-19 in different organs, especially on the central nervous system, kidneys, and the gastrointestinal tract. The authors also recommend that more studies are conducted in areas with higher prevalence of comorbidities, such as viral hepatitis or tuberculosis, so that the effects of specific pre-existing conditions on the outcome of the virus can be determined. Studies comparing the effects of COVID-19 on various organs in healthy patients versus in those with pre-existing conditions will also be helpful.
CONCLUSION
In conclusion, the many findings in multiple organs could not be definitively attributed to SARS-CoV-2 as many patients had underlying pre-existing conditions; therefore, further case- control type studies are required to credit the histopathological findings to SARS-CoV-2.








