Abstract
Urinary tract infection (UTI) in children is one of the most common bacterial infections that propels inappropriate antibiotic use. Long-term, potentially fatal complications can occur if not properly treated. Prompt investigation and appropriate treatment would prevent these complications. Although urine culture remains the gold standard investigation for UTI, its process is cumbersome and requires time (24–72 hours). Hence, there has been growing interest in the use of urinary biomarkers. However, some conventional urinary biomarkers detected on urinalysis have poor sensitivity values when used singly as a screening tool. Thus, the searchlight has shifted to the role of novel biomarkers in UTI diagnosis. This narrative review aimed to determine if elevated levels of these biomarkers directly correlate with positive urine cultures. A positive correlation may imply that these biomarkers could serve as novel UTI diagnostics and thus augment urine culture requests. Established and recent serum and urinary biomarkers show disparate predictive abilities for UTI and its related complications. Some have elevated differential levels in upper and lower UTI or febrile and non-febrile UTI. All studies that investigated these biomarkers established culture-positive UTI, highlighting a direct correlation between positive urine cultures and increased concentrations of the biomarkers in body fluids. Because certain uropathogens were less likely to be associated with pyuria, the sensitivities of some neutrophil-related novel biomarkers (such as urine neutrophil gelatinase-associated lipocalin and human neutrophil peptides 1–3) were reduced in cases of UTI caused by these bacteria. While levels of these novel biomarkers directly correlate with positive urine cultures, it appears that there is yet no standalone biomarker with the optimal sensitivity and specificity for UTI. Although these novel biomarkers are promising, translating their measurements into clinical practice with specific clinical utilities will take time. Novel methods interrogating high-throughput serum (and urine) metabolome data with positive urine cultures in a platform-agnostic manner (metabolome-wide approach) will help confirm and identify novel biomarkers that might capture specific aetiologic agents or shared pathways of related agents. The authors recommend that future research on UTI diagnostics should specifically focus on identifying highly sensitive and specific standalone novel biomarkers that can be easily applied as a point-of-care investigation.
INTRODUCTION
Urinary tract infection (UTI) refers to any infection of the upper urinary tract (pyelonephritis) or the lower urinary tract (cystourethritis) by uropathogens and is one of the most common bacterial infections that propels inappropriate antibiotic use in children. Thus, UTI contributes significantly to childhood morbidity and mortality.1 For instance, acute pyelonephritis may result in renal parenchymal scars in approximately 10–30% of children with febrile UTI if not well treated.2 The clinical trajectory of these scars comprises hypertension, chronic kidney disease, and eventually, end-stage kidney disease.3 To circumvent these complications, any suspected case of UTI in a child should be promptly investigated and adequately treated.
The gold standard for investigating childhood UTIs remains urine culture. The definition of positive urine culture is predicated upon specified colony counts of a uropathogen cultured from urine specimens obtained by different methods from a paediatric patient. The paradigm for UTI diagnosis is generally accepted as the growth of a single uropathogen at a concentration of >103 colony-forming units (CFU)/mL, >104 CFU/mL, or ≥105 CFU/mL from urine specimens obtained by suprapubic aspiration (SPA), catheterisation, and midstream clean-catch, respectively.4 However, the American Academy of Pediatrics (AAP) revised guideline for childhood UTI proposes a combination of bacteriuria with or without pyuria and bacterial growth of ≥5×104 CFU/mL of a single uropathogen from either SPA or catheter urine specimens as the diagnostic parameters.5 Interestingly, the AAP recommendation differs from the guideline put forward by the National Institute for Health and Care Excellence (NICE). The latter recommends the combination of urinary biomarkers (such as nitrite and leukocyte esterase) and colony count of any Gram-negative bacillus or >103 CFU/mL of a Gram-positive coccus (from SPA urine specimen), or >104 CFU/mL of a single uropathogen (from a catheter urine specimen), or ≥105 CFU/mL of a single uropathogen (from a midstream urine specimen).6
Given the different positions adopted by the two guidelines on the diagnostic utility of some conventional urinary biomarkers (like pyuria, nitrite, and leukocyte esterase), their suboptimal predictive value or sensitivity when used alone has necessitated the searchlight on novel biomarkers as alternative diagnostic tools.7 These biomarkers have been found helpful in the diagnosis of UTI,8-10 in predicting its complications,11-14 and in discriminating between febrile UTI (acute pyelonephritis) and lower UTI.15
Based on published evidence, a positive correlation of a biomarker with positive urine culture may imply its diagnostic utility and augmentation with urine culture. Therefore, this narrative review summarises the novel biomarkers and the extent to which they capture positive urine cultures. Relevant descriptors (“urinary tract infection,” “children,” “novel biomarkers,” “urine culture,” and “correlation”) were used. The authors searched two electronic databases (PubMed and Google Scholar) for relevant articles published in English or translated into English. Additional information was gleaned from any review articles published on the subject.
ESTABLISHED NOVEL BIOMARKERS IN URINARY TRACT INFECTION DIAGNOSTICS
Although several novel biomarkers have now been adjudged potentially valuable for the diagnostic evaluation of bacterial UTI in children, procalcitonin and ILs are prominent among them.7 For instance, the uropathogenic Escherichia coli (including other Gram-negative bacilli and Gram-positive cocci) interacts with the uroepithelial cells and the peripheral blood monocytes and triggers the pathophysiologic pathways that result in elevated levels of these biomarkers in urine and serum, respectively (Figure 1). First, the infecting strain of E. coli is equipped with P fimbriae, which promotes its adherence to the mucosal lining of the urinary tract. After the uropathogen has attached to the uroepithelial cells, an inflammatory response occurs, including IL-6 and IL-8 secretion.16 While IL-6 elicits the febrile and acute phase responses seen in acute pyelonephritis, IL-8 facilitates chemoattraction of neutrophils to the urinary tract mucosal site. The build-up of neutrophils contributes to the onset of pyuria. The mucosal IL-8 response triggered by E. coli underscores the significant contribution of the uroepithelial cells to the IL-8 levels during UTI.17 Second, an in vitro study showed that the same uropathogen also stimulates the peripheral blood monocytes to produce IL-1α, IL-1β, IL-6, IL-8, and TNF-α.18 These proinflammatory cytokines (especially IL-1β, IL-6, and TNF-α) then send signalling information to the parafollicular (C cells) and neuroendocrine cells to produce procalcitonin. Its level rises in response to this bacteria-induced proinflammatory stimulus and represents an acute phase reactant, as well as a biomarker of severe bacterial infection.19 Fundamentally, a bacterial infection induces a substantial increase in the expression of the gene encoding calcitonin 1, leading to the production of procalcitonin in all of these differentiated cell types.20
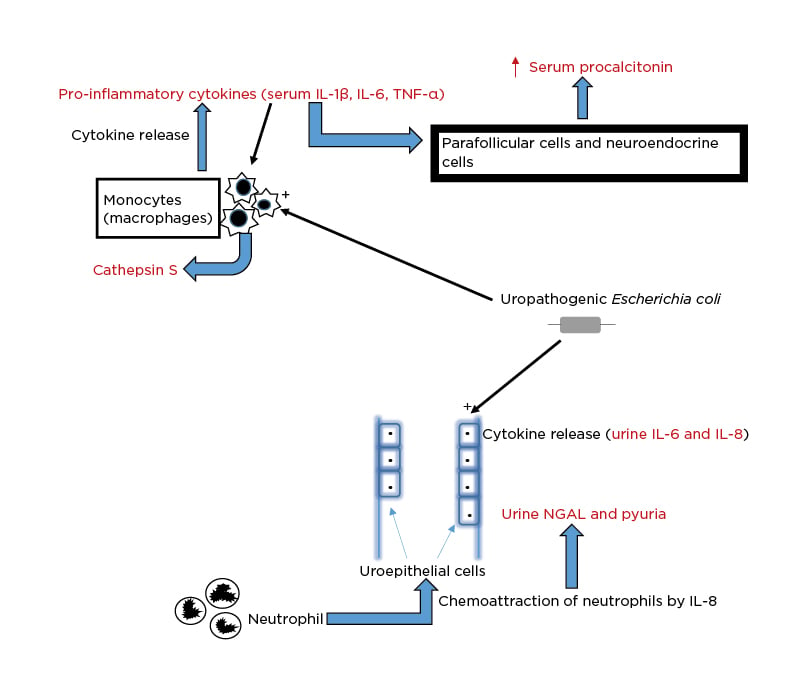
Figure 1: Schematic illustration of the pathophysiologic basis for the elevation of biomarkers in
urinary tract infection.
NGAL: neutrophil gelatinase-associated lipocalin.
Several published reports indicate that procalcitonin and ILs are reliable biomarkers in diagnosing UTI in children. In one observational analytical study, the investigators noted higher levels of IL-6 and IL-8 in the serum and urine of children with febrile UTI than in those of their cohorts with asymptomatic bacteriuria.21 They also observed that urine IL-6 and IL-8 levels directly correlated with urine leukocyte counts (pyuria) and acute phase reactants like C-reactive protein (CRP) and erythrocyte sedimentation rate. The children’s age, gender, and bacterial properties (such as the possession of P fimbriae) also influenced the urine levels of these cytokines. Thus, it is apparent that host- and bacteria-related factors affect cytokine responses in UTI and the presence of pyuria. Some studies reported a lower risk of pyuria in childhood UTI aetiologically attributed to Enterococcus species, Klebsiella species, and Pseudomonas aeruginosa, and a higher risk of pyuria in uropathogenic E. coli-related cases.22
In a related comparative study, both serum and urine IL-6 and IL-8 levels were significantly elevated in children with acute pyelonephritis than in their counterparts with lower UTI as well as in uninfected controls.23 Serum and urine IL-6 exhibited higher sensitivity and specificity in diagnosing acute pyelonephritis than serum and urine IL-8. This finding was corroborated in another study, where infants with acute pyelonephritis had higher serum IL-6 levels than their cohorts with lower UTI.24 These findings underscore the crucial role of IL-6 in the upregulation of serum procalcitonin production seen in acute pyelonephritis and their discriminatory capacity for upper and lower UTIs.
More importantly, there is consistent evidence across several studies indicating that serum procalcitonin levels were significantly higher in children with acute pyelonephritis than in those with lower UTI.25-28 Additionally, some studies demonstrated the prognostic utility of this biomarker for UTI. High serum procalcitonin levels were strongly predictive of high-grade vesicoureteral reflux in children with the first episode of febrile UTI.11,29,30 The confirmation of this finding precludes the use of imaging studies in low-risk patients. Elevated serum procalcitonin strongly predicted the presence of renal scars in children with culture-proven UTI seen at admission and was even more specific than CRP and pyuria in identifying those children at risk for this complication.31 Similarly, a hospital-based prospective study showed that serum procalcitonin was an early predictor of renal parenchymal damage in children with the first episode of febrile UTI.32 This observation makes the biomarker a reliable tool for prognostication in UTI.
Finally, urine neutrophil gelatinase-associated lipocalin (NGAL), urine IL-1β, and urine 8-hydroxy-2’-deoxyguanosine (8-oxodG) are other novel biomarkers that are useful in the diagnosis and prognosis of childhood UTI.14,33,34 Whereas urine NGAL exhibited high sensitivity for diagnosing UTI,33 urine 8-oxodG predicted renal parenchymal damage in children with UTI.14 Furthermore, urine IL-1β was a valuable biomarker for the early diagnosis of febrile UTI in infancy and late childhood.34 In summary, the novel biomarkers currently found reliable in diagnosing and prognosticating UTI in children include serum procalcitonin, urine/serum IL-6 and IL-8, urine NGAL, urine IL-1β, and urine 8-oxodG. Serum procalcitonin is particularly useful in predicting UTI complications (such as vesicoureteral reflux) and as a marker of febrile UTI severity, while urine/serum IL-6 and serum procalcitonin can be used to differentiate upper UTI from lower UTI. All studies that investigated these biomarkers established culture-positive UTI, highlighting a direct correlation between positive urine cultures and increased concentrations of the biomarkers in body fluids.
MORE RECENT NOVEL BIOMARKERS IN URINARY TRACT INFECTION DIAGNOSTICS
In the past few years, more novel biomarkers (significantly elevated in culture-proven UTI) are still emerging and are currently being investigated. Given the accumulating evidence on the utility of novel biomarkers in UTI diagnostics, there could be a paradigm shift to incorporate the microbiological evaluation (positive urine cultures) with biochemical evaluation (elevated serum/urine novel biomarkers) if there is a strong correlation between positive urine cultures and raised levels of these novel biomarkers. However, the diagnostic paradigm would have to be validated by scientific data that support this direct association.
In a recent prospective cohort study of children with culture-positive UTI and those with culture-negative pyuria, cathepsin S (CTSS) levels were elevated in the urine of children with the former when compared with the urine of children with the latter.35 CTSS is a lysosomal enzyme expressed by antigen-presenting cells such as macrophages (mononuclear phagocytes) and B lymphocytes (Figure 1). Immune cells (including macrophages) secrete CTSS in response to inflammatory mediators such as proinflammatory cytokines and neutrophils. In the bacterial infection of the urinary tract, the mononuclear phagocytic system involved in innate immunity produces this novel biomarker (CTSS), which subsequently appears in the urine and could, therefore, be regarded as a surrogate of culture-positive UTI.
The diagnostic performance of urine NGAL in childhood UTI has been previously discussed. Another recent study has underscored its superiority over other biomarkers, including CRP and procalcitonin, in distinguishing between acute bacterial and viral infections.36 The authors demonstrated human neutrophil lipocalin or NGAL levels in serum or whole blood activated by formyl-methionine-leucine-phenylalanine and showed this discriminating ability with high accuracy. Furthermore, the procedure is suggested as a potentially acceptable point-of-care investigation due to its response time of less than 15 minutes.36 Thus, a raised serum NGAL in children with UTI predicts a bacterial aetiology and suggests a possible concordance with a positive urine culture. Serum NGAL has been reported as an ideal biomarker for the early diagnosis of culture-proven febrile UTI (acute pyelonephritis), with sensitivity and specificity higher than those of urine NGAL.37 A recent report suggests that the predictive values and likelihood ratios of urine NGAL were superior to those of serum CRP and white blood cell count for detecting febrile and non-febrile UTI at each cut-off level.38 However, urine NGAL levels are influenced by the urine sampling technique. Bagged urine samples exhibited lower quantitative and dipstick NGAL specificities than catheterised urine samples.39 Other studies also established that urine NGAL expression temporally correlates with the bacterial stimulus, responds in a quantitative trend to the bacterial colony count, and rapidly reverses with the resolution of infection.40-42
Furthermore, antimicrobial peptides (human α-defensin 5 [HD5] and human neutrophil peptides 1–3 [HNP1–3]) were recently evaluated for their diagnostic accuracy in children with culture-positive UTI.43 HNP1–3 is a neutrophil-related biomarker and indicates the presence of pyuria. In contrast, HD5 is expressed throughout the urothelium of the lower urinary tract and in the nephron and renal collecting tubules. In order of frequency, the bacterial isolates accounting for positive urine cultures included E. coli, Staphylococcus saprophyticus, Klebsiella pneumoniae, and Serratia marcescens. Urinary HD5 and HNP1–3 levels were significantly higher in culture-positive urine samples than culture-negative urine samples. When combined with leukocyte esterase (detected on urinalysis), HD5 significantly increased specificity without decreasing sensitivity, especially when the urine sample was collected by the clean-catch method.43 These findings highlight, to some extent, the diagnostic utility of the biomarkers and their dependence on the urine-sampling method.39 Urine HD5 and NGAL are more likely to correlate with UTI aetiologically attributed to E. coli than with UTI from other bacterial agents since pyuria frequently occurs in the former.22 However, there is no consensus on the diagnostic value of these two biomarkers as they also exhibited poorer diagnostic utility in another study.44
In a recent comparative study, 30 children with culture-positive febrile UTI and 30 healthy controls were investigated for the diagnostic value of soluble triggering receptor expressed on myeloid cells-1 (sTREM-1) as a novel biomarker for childhood UTI.45 sTREM-1 had a sensitivity of 57% and a specificity of 50% for the diagnosis of UTI. TREM-1 is a protein surface receptor that amplifies toll-like receptor-induced inflammation by increased production of proinflammatory cytokines.46 Its soluble form (sTREM-1) accumulates in the bloodstream during inflammation and has been used to provide valuable information on the degree and outcomes of inflammatory processes in sepsis and pneumonia,47 as well as inflammatory bowel disease.48 sTREM-1 does not have a high sensitivity and specificity compared with previously established biomarkers like serum procalcitonin and NGAL. However, bacterial infection is an essential factor that increases TREM-1 expression, providing reliable evidence that positive urine culture most likely correlates with this novel biomarker.
Serum calprotectin (sCAL) is another potentially predictive biomarker of bacterial UTI in febrile children.49 sCAL is a heterodimer consisting of S100A8 and S100A9 proteins from the family of S100 proteins, with suggested clinical value as a biomarker of inflammation. It has now been investigated as a possible biomarker for bacterial UTI in children. Among 22 children with culture-positive UTI, sCAL showed a sensitivity of 93.1% and specificity of 76.2%. Thus, sCAL levels in febrile children could be an additional biomarker of consideration in the prompt and accurate diagnosis of UTI.49 This lends credence to the role of inflammatory biomarkers as potential predictors and biomarkers of UTI.
Another recent study investigated the serum levels of oxidative stress biomarkers such as malondialdehyde (MDA) and total antioxidant capacity (TAC) in children with culture-positive UTI.50 Oxidant–antioxidant balance biomarkers (MDA and TAC) were quantified in their serum samples. Urine culture was performed to identify the causative organism of UTI. Overall, a significant elevation in serum MDA and a decrease in serum TAC was found. E. coli was the most frequent bacterial aetiologic agent for UTI in these children. It was concluded that the rise in serum MDA and reduction in serum TAC levels also implied an attenuation in the protective effects of antioxidants, given that low levels of reactive oxygen species are typically produced during tissue metabolism.50
Table 1 summarises the potential indications of the established and more recent novel biomarkers. Beyond their utility in UTI diagnostics,53 they have hitherto been found useful in various other diseases.51,52,54 Obviously, these biomarkers are highly sensitive and specific in detecting bacteria-induced inflammatory diseases.
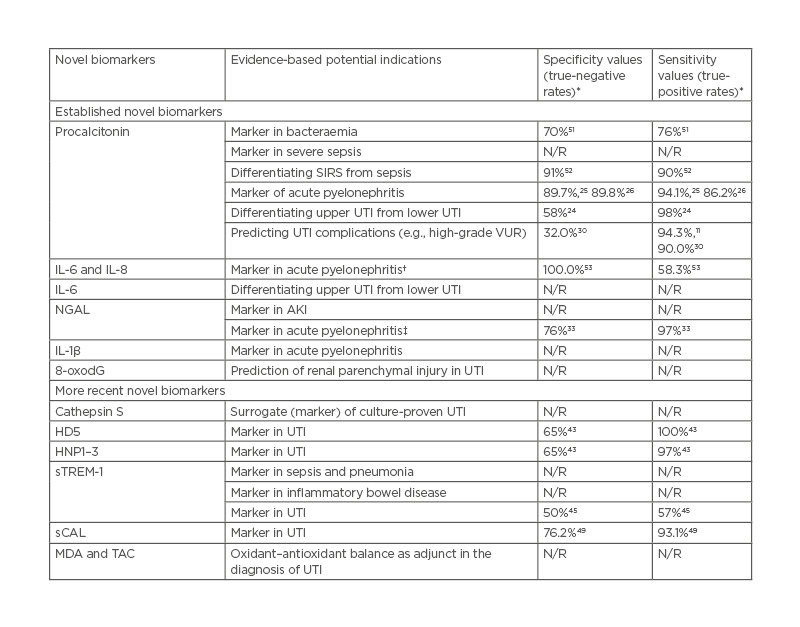
Table 1: Potential indications and sensitivity/specificity values of established and more recent novel biomarkers in urinary tract infections.
*Values reported in the referenced studies.
†Serum/urine IL-6 more sensitive and specific than serum/urine IL-8.
‡Serum NGAL more sensitive and specific than urine NGAL.
AKI: acute kidney injury; HD5: human α-defensin 5; HNP1–3: human neutrophil peptides 1–3; MDA: malondialdehyde; NGAL: neutrophil gelatinase-associated lipocalin; N/R: not reported; sCAL: serum calprotectin; SIRS: systemic inflammatory response syndrome; sTREM-1: soluble triggering receptor expressed on myeloid cells-1; TAC: total anti-oxidant capacity; UTI: urinary tract infection VUR: vesicoureteral reflux; 8-oxodG: 8-hydroxy-2′-deoxyguanosine.
CONCLUSIONS AND FUTURE RESEARCH DIRECTIONS
Identifying novel biomarkers that are more accurate than conventional biomarkers (e.g., nitrite or leukocyte esterase) may help to enhance UTI diagnosis and minimise inappropriate antibiotic treatment in children with the infection. Thus, novel UTI diagnostics hold prospects as alternative tools for improved management of UTIs. This review has shown that established and more recent serum and urinary biomarkers exhibit disparate predictive and diagnostic abilities for UTI and its related complications. Some have elevated differential levels in upper and lower UTI (or febrile and non-febrile UTI), and thus could be used to distinguish between the two forms of UTI. Their sensitivity and specificity values vary from moderate to high percentages. Most studies reporting their diagnostic value also documented culture-positive UTIs in the evaluated children. Because certain uropathogens are less likely to be associated with pyuria, the sensitivities of some neutrophil-related novel biomarkers (such as urine NGAL and HNP1–3) are reduced in cases of UTI caused by these bacteria. While levels of these novel biomarkers directly correlate with positive urine cultures, it appears that there is no single biomarker with the optimal sensitivity and specificity. Worse still, the shared pathophysiological mechanism of other bacterial infections in producing biomarkers (such as procalcitonin) makes it challenging to have a specific biomarker for UTI. However, some biomarkers’ sensitivity and specificity for UTI are augmented with conventional biomarkers like leukocyte esterase. Although these novel biomarkers are promising, translating their measurements into clinical practice with specific clinical utilities will take time. On the other hand, novel methods interrogating high-throughput serum (and urine) metabolome data with positive urine cultures in a platform-agnostic manner (metabolome-wide approach) will help confirm and identify novel biomarkers that might capture specific aetiologic agents or shared pathways of related agents. Thus, future research on UTI diagnostics should specifically focus on identifying highly sensitive and specific standalone novel biomarkers that can be easily applied as a point-of-care investigation.







