Abstract
Background: The 2019 novel coronavirus (COVID-19) pandemic resulted in significant mortality and morbidity. Ursodeoxycholic acid (UDCA) is reportedly widely in demand in some countries, such as China, to protect individuals from the effects of infection, as there is evidence that it is effective in preventing viral replication in some in vitro studies. UDCA is commonly prescribed in patients with primary biliary cirrhosis and gallbladder calculi. By evaluating a set of patients prescribed UDCA, whether or not the risk of COVID-19 infection is attenuated by adherence to UDCA can be determined.
Method: This is a retrospective database study using the Clinical Practice Research Datalink (CPRD Aurum). Patients who received a prescription of UDCA in the study timeframe of March 1, 2020–May 30, 2021 were characterized, and their primary care electronic medical records analyzed for presence of COVID-19 infection. The proportion of days covered for each patient was used as a proxy for adherence. A comparison was made between categorized high- and low-adherence, and adherence as a continuous variable. Inverse probability weighting was used to adjust for confounding.
Results: Higher categorized adherence (≥80%) to UDCA was associated with a statistically significant lower incidence of COVID-19 (odds ratio [OR]: 0.864; 95% confidence interval [CI]: 0.759–0.984; p=0.027). This contrasted to adherence as a continuous variable, which was not statistically significant. Obesity and hematological malignancy were also associated with a higher incidence of COVID-19 infection.
Conclusion: There is evidence to suggest that the regular use of UDCA is associated with a lower risk of COVID-19 infection when compared to irregular or sporadic usage.
Key Points
1. A high medication adherence to ursodeoxycholic acid appears to confer protection against COVID-19 infection, as reported by primary care coding from the start of the COVID-19 pandemic to the second quarter of 2021, which supports existing literature from prospective and in vitro studies.
2. The relationship between ursodeoxycholic acid prescription and positive primary care coding for COVID-19 infection appears to be non-linear, indicating that little protection is conferred by intermittent or sporadic prescription/dosing.
3. The possibility of an easily tolerated and inexpensive prophylactic medication could provide an alternative treatment for those who do not qualify for existing prophylactic treatments, or who do not tolerate vaccination, but still have a higher risk of morbidity from COVID-19.
INTRODUCTION
The 2019 novel coronavirus (COVID-19) pandemic has resulted in significant mortality and morbidity worldwide. Around the world, around 650 million cases of infection, and around 6.6 million related deaths had been reported by the World Health Organization (WHO) by December 23, 2022. During the pandemic, governments throughout the world instituted lockdowns of varying length and strictness, widespread testing and, eventually, mass vaccination programs.1 A significant number of therapies were adapted or purposefully created for the prophylaxis and treatment of patients suffering from COVID-19.2 Tixagevimab and cilgavimab, a combination of two monoclonal antibodies, were recommended in the UK in 2022 for the prevention of COVID-19 in those who had weakened immune systems and were unable to have a vaccination, or who were unlikely to mount an immune response to vaccination.3 In addition, four antiviral medications (nirmatrelvir, ritonavir, remdesivir, and molnupiravir) are available to those with COVID-19 who are at the highest risk of becoming ill. Other medications, such as hydrochloroquine4 and favipiravir,5 have also been explored, but evidence is lacking for efficacy.
Ursodeoxycholic acid (UDCA) has been investigated as a drug with the potential to treat COVID-19.6 UDCA is prescribed for dissolution of small gallbladder calculi in patients with asymptomatic cholelithiasis, or those with primary biliary cirrhosis.7,8 It is generally safe and well tolerated.9,10 UDCA has potential as a low-cost prophylactic and treatment for COVID-19. It is prescribed in secondary and primary care, and has a half-life of 3–6 days. Typical dose ranges from 300–900 mg/day, dependent on bodyweight.11
The protective mechanism of UDCA for COVID-19 has been attributed to the interaction with angiotensin-converting enzyme 2 (ACE2) receptor. Research has indicated that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) gains entry into target cells by binding its spike protein to the ACE2 receptor. ACE2 maintains a strong affinity for COVID-19, and its expression remains unaltered despite viral mutations. A recent investigation has highlighted the potential of UDCA, a medication primarily employed to treat liver conditions, in thwarting COVID-19 infection by diminishing ACE2 expression.12 Subsequent validation of this discovery has been achieved through both in vitro and in vivo experiments.13
The current arsenal of antibodies generated by vaccination struggles to effectively neutralize the virus, due to its adept immune evasion tactics. Additionally, the concentration of antibodies declines notably over time, a phenomenon exacerbated in older adults and high-risk populations, such as patients who are immunocompromised, who exhibit reduced responsiveness to vaccines.14 In light of these challenges, the development of novel therapeutic agents targeting alternative aspects of the disease becomes imperative. This approach holds promise in mitigating both the risk and severity of the illness, offering particular benefits to individuals ineligible for monoclonal antibody treatment, those lacking access to vaccination, and individuals with underlying health conditions who share close quarters with individuals infected with COVID-19.
As of writing, public concern about COVID-19 has waned,15 with COVID-19 restrictions lifted in the UK in February 2022.16 As a contrast, China has had a zero COVID-19 policy in place since the pandemic emerged in 2020, with restrictions only recently lifted at the end of 2022.17 There has been a reported scramble over the counter for UCDA in China, due to less effective vaccines, lower than ideal vaccination in the elderly population, and significantly fewer intensive care beds per head of population than the USA.18 In a recent study published at a single center, the Beijing Ditan Hospital in China, UDCA was associated with fewer and milder COVID-19 infections based on self-reporting in patients with chronic liver disease during 2022.19 This study aimed to determine whether dosages of UDCA are associated with the risk of COVID-19 infection in the UK, in a select population of patients prescribed UDCA.
METHOD
The authors initiated a retrospective database study, broadly covering the first reports of COVID-19 being transmitted in the UK, around March 1, 2020, to the reopening after the second lockdown in the UK, May 30, 2021.
A single cohort of patients was followed from the index date (the start of the study period) to the end of the study period. A baseline period prior to the index date was utilized to evaluate comorbidities of a patient, and to evaluate the length of the previous UDCA prescription.
Two populations were examined: patients with documented chronic liver disease, and patients without, who have been presumed to be prescribed UDCA for gallbladder calculi. As the drug is not approved for COVID-19 prophylaxis or treatment, the population that has been examined in this study have been prescribed UDCA for indications such as asymptomatic cholelithiasis or primary biliary cirrhosis. This study took place from the first corresponding, to the creation and notification to primary care physicians of COVID-19 specific codes, the lifting of the first lockdown, to the reopening of indoor venues in the UK.20 The start of the period was limited by the provision of codes to record a positive COVID-19 diagnosis.
As this was a real-world retrospective study, a surrogate for the dosage of UDCA was required. The proportion of days covered (PDC) is frequently used as measure of adherence to medications in real-world databases.21 The comorbidities and cofactors were based on comorbidities listed by the UK’s National Health Service (NHS) as increasing the risk of COVID-19 infection.22 In this study, the authors utilized the PDC as a surrogate for the dosage of UDCA over the study period. UDCA prescriptions are presumed to not expire during the study period; hence, two prescriptions lasting 30 days will result in 60 days medication coverage. The patient is also assumed to have used the medication as directed. As patients with long-term prescriptions are likely to have coverage from prior to the study period, the prior prescription will also be considered in the calculation of PDC.
Data Source
The authors’ retrospective cohort study used Clinical Practice Research Datalink (CPRD Aurum), an ongoing primary care database of anonymized medical records from general practitioners, which comprises over 40 million research-acceptable patient records (permanently registered with sufficient data quality).23,24 The database covers over 20% of the UK population. The CPRD primary care database is therefore a rich source of health data for research, including data on demographics, symptoms, tests, diagnoses, therapies, health-related behaviors, and referrals to secondary care. Each year, CPRD must obtain Section 251 regulatory support through the Health Research Authority (HRA) Confidentiality Advisory Group (CAG). All requests from researchers to gain access to linked data must be approved via the CPRD Research Data Governance (RDG) Process.
Exposure Studied
The exposure studied was the adherence, as measured by the PDC for UDCA. Prescription duration for a single prescription of UDCA was calculated by the prescription length in days entered by the physician. Where prescription length was recorded as 0 days, the median prescription duration was imputed by the associated prescription instructions in the dosage text, or if unavailable, the mean length in days for that particular prescription. Medication supplied from prescriptions filled before the end of the previous supplies was also carried forward to after the end of the previous days supplied.
Population
The population of the study included patients who fit the eligibility criteria at the index date (March 1, 2020). Patients who were only prescribed UDCA after the index date were not included.
Inclusion Criteria
Eligible patients included those who had a prescription issued for UDCA in the 6 months prior to the study period. Patients exited the study when they were recorded as having a date of death, or were unregistered from a CPRD-participating practice.
Outcome Assessments
Presence of code for COVID-19 infection, either through a clinical diagnostic code, or a test result indicating a positive COVID-19 infection.25
Patient Demographics
After applying the inclusion and exclusion criteria, 8,964 patients were identified. The mean age of the low-adherence group was lower than the high-adherence group (57 versus 68; p<0.001). The proportion of the low-adherence group who were male was lower than the high-adherence group (69% versus 73%; p<0.001).
Power Study
With a proportional outcome and using a type I error rate of 0.05 and a power of 70%, with a higher number of low-adherence patients to adherent patients (a ratio of 1.3), event rate of 0.059 in the low-adherence group, and event rate in the high-adherence cohort of 0.047. The required sample size was 3,837 in the high-adherence group, and 4,989 in the low-adherence group. Within the data fitting the eligibility criteria, there were 3,904 patients in the high-adherence group, and 5,060 in the low-adherence group, indicating that there were enough patients to sufficiently power the study.
Statistical Analysis
Descriptive statistics are presented as proportions and means with standard deviation or, where categorical, median with interquartile ranges. Adherence was considered as a categorical variable for descriptive purposes where poor adherence included patients with a PDC of <80%26 and high-adherence as PDC ≥80%. Adherence was also considered as both a continuous variable and a categorical variable for the outcome analysis. The objectives of comparing risk of COVID-19 documented infection will be modeled through binary logistic regression. The odds of COVID-19 diagnosis or test/diagnosis versus no diagnosis or test/diagnosis in patients who were adherent (high-adherence, or PDC >80%) were compared to patients who were non-adherent (low-adherence, or PDC <80%). To account for confounding between the subcohort of patients with high-adherence and low-adherence, inverse propensity weighting was used to enable balance. This method was chosen because of the relatively low number of COVID-19 infections recorded, and the unwillingness of the authors to discard patients. COVID-19 vaccination was considered as a time dependent variable. The significance level was set to 5%.
All statistical analysis was performed using R Statistical Software (version 4.1.1; R Foundation for Statistical Computing, Vienna, Austria). In the study population, 482 patients had a record of COVID-19 infection within primary care. There were 3,904 patients with low-adherence <80%, and 5,060 with higher adherence. There were a total of 1,657,469 follow-up days in the high-adherence subcohort, with a mean follow-up of 425 days. For the low-adherence subcohort, there were a total of 2,162,435 follow-up days, with a mean follow-up of 427 days. The median time of follow up was 426 days. Within the two groups there were significant differences, shown in Table 1. Statistically significant differences were seen in age, sex, use of immune suppressants in the baseline year, solid tumor, hemato-oncology conditions, chronic obstructive pulmonary disease, smoking status, serious liver disease, chronic renal failure, and rheumatological condition. There were no statistically significant differences in the proportion with BMI ≥30 kg/m2, asthma, or chronic kidney disease between the two groups. It is worth noting that the proportion of COVID-19 was slightly higher in unmatched patients who had a higher adherence compared to those with a low adherence.
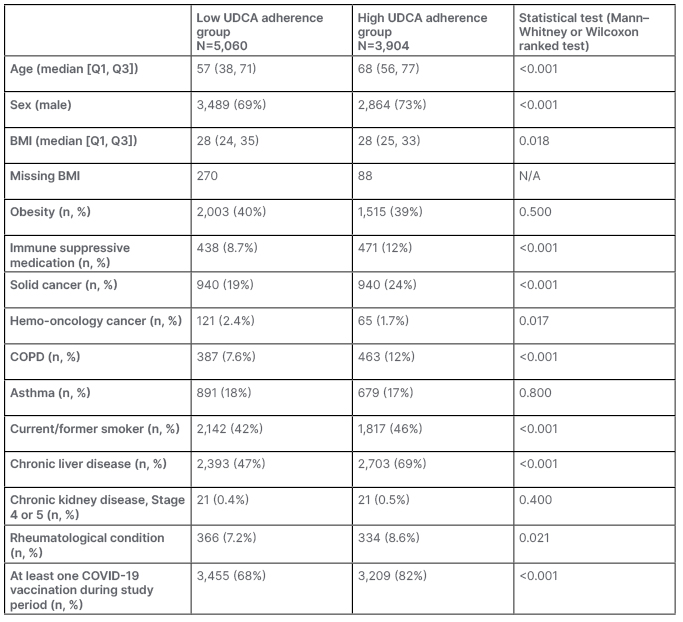
Table 1: Clinical characteristics of patients prescribed ursodeoxycholic acid.
COPD: chronic obstructive pulmonary disease; N/A: not applicable; UDCA: ursodeoxycholic acid.
Seventy-four percent of the study population had a code for COVID-19 vaccination by the end of the study period. There were 561 deaths and 723 people lost to follow-up, including to death.
Within the low adherence group, the number of patients with a COVID-19 code was 297, which equates to 5.9% of the subcohort. Within the high adherence group, the number of patients with a COVID-19 code was 185, which included 4.7% of the group. In a non-weighted, non-adjusted comparison, the odds ratio (OR) was 0.81.
After weighting each of the patients according to their demographics and comorbidities, the analysis was repeated with results shown in Table 2. Adherence as a continuous variable was not a statistically significant explanatory variable affecting COVID-19 coding in the multivariable model (p=0.55). The OR of a positive COVID-19 code was 0.998 (95% CI: 0.997–1.000; p=0.244). After appropriate weighting, adherence as a categorized variable consistent of <80% adherence and ≥80% adherence was significant, with an OR of 0.864 (95% CI: 0.759–0.984; p=0.027). The direction of effect is in favour of persons in the higher adherence group being at lower risk of receiving a code for COVID-19 in primary care. Additionally, a multivariable model examining other candidate variables that may influence the number of COVID-19 codes was analyzed. From demographics and comorbidities, only obesity (p<0.001) and hematological malignancy (p=0.041) were significant, as shown in Table 3.
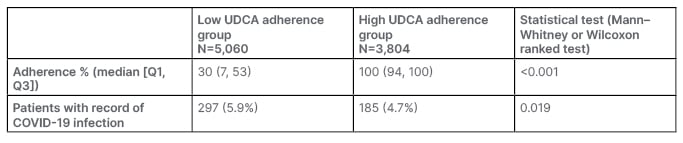
Table 2: Adherence and COVID outcomes (note: adherence was capped at 100%).
UDCA: ursodeoxycholic acid.
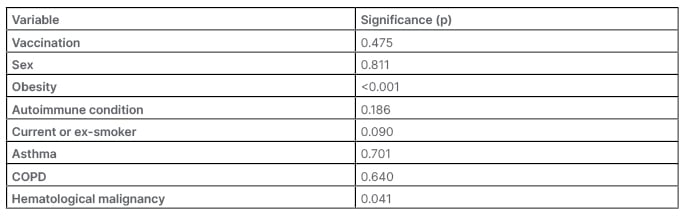
Table 3: Multivariable regression for covariates associated with the outcome of COVID-19 infection code in primary care.
COPD: chronic obstructive pulmonary disease.
DISCUSSION
In the authors’ limited study population, categorized higher adherence to UDCA was associated with a lower incidence of COVID-19 infection. Of note, no patients were identified with a diagnosis of sickle cell anemia, despite an increased association with gallstone formation.26 This would be consistent with speculation that high adherence to UDCA has the potential to be protective to COVID-19 infection. The conclusions drawn from this retrospective study are consistent with Li et al.’s19 2023 prospective study, and the lower incidence of COVID-19 as assessed by telephone reporting. The groups reported in Li et al.’s19 study were patients who had reported 1 or more months of UDCA prescription, and those who were not receiving UDCA.
The lack of significant association with adherence as a continuous variable may initially appear to counter the hypothesis that UDCA is protective; however, the lack of significance may show that protective effects only occur with patients who were highly adherent. Since the cut-off between low- and high-adherence groups was 80% coverage, there was a significant difference between the two groups, with the median adherence of >100% in the high group, compared to 30% in the low-adherence group. Again, this was consistent with Li et al.’s19 study, where patients not receiving UDCA were compared to those who were. There are many confounding factors that may contribute to this protective effect that could contribute to patients being more adherent, including various psychosocial factors, adaptive strategies, and personal difficulty in life situations.27 Other confounding factors that are known to affect both COVID-19 infection and adherence, such as social deprivation, education, financial status, and partnership, are also not accounted for in this study.28,29 Issues around access to medication were magnified during this period, and included mandatory self-isolation, changes to the way prescriptions were ordered and collected, and worries around contact with COVID-19.30 During this period, there were significant COVID-19 precautions, including three national lockdowns in England, regional lockdowns, mask wearing in public, and a program of vaccination with vaccines with different effectiveness. Although some relevant risk factors are included in the analysis, many of these factors are not taken into account. Patients who have a better prescription coverage have better organization and planning,31 which may help adherence to social distancing, and isolation methods that may reduce risk of infection. Patients who adhere to their medication tend to be patients who suffer from more severe disease,32 and may be more likely to self-isolate.
In this study, the authors have used prescription coverage as a surrogate for adherence and medication use. This assumption requires both prescription coverage to represent prescription collection, and for patients who collect prescriptions to take them as directed. There can be situations where physicians issue prescriptions, but there are barriers to collection. During the pandemic, there were significant changes in how primary care was accessed, which may have resulted in lower prescription pick-up. The reduction in face-to-face consultations over this period could have also resulted in a lower actual adherence compared to prescription rate, as it has been shown that face-to-face consultations are beneficial to promote medication coverage.33 There are, however, limitations to this consideration, since patients can be prescribed medication, but do not collect it from the pharmacy. Additionally, patients can receive medication from a pharmacy, but do not take their prescription as prescribed. Stockpiling pharmaceutical medications was observed during the pandemic, and thus many people requesting or collecting medications may have demonstrated this behaviour.34 Despite these limitations, records of prescription are widely used as a surrogate for adherence in chronic disease.35
Structural limitations exist within the study design, including the limitation of only collecting data within primary care. Many medication codes were only implemented into electronic medical record systems in June 2020, and thus do not record cases of COVID-19 prior to this timeframe. The adoption of the use of the codes by users of electronic medical record systems is unknown. COVID-19 recorded by home testing kits and not reported to the GP, COVID-19 recorded in secondary care, and testing centers are not recorded. COVID-19 vaccinations are likely to be incompletely recorded in primary care, given that many vaccinations took place in vaccination centers. The type of vaccination was also inconsistently recorded, and was not considered as a variable. There may be variation in the effectiveness of monovalent and bivalent vaccinations, and in the number of different vaccinations patients receive.36 This study only measures occurrences of COVID-19, and does not measure severity, or the presence or absence of any protective effect to severe disease. Hospital attendances and deaths due to COVID-19 are not recorded.
Future avenues for research include utilisation of hospital records to augment primary care records. This would allow the distinction of COVID-19 infections that were dealt with inside the community, those that required hospitalisation, and those that required intensive care. If numbers allowed, length of stay could also be assessed. This would allow evaluation of whether UDCA prevented hospitalisation and intensive care unit admission, and also assess whether the length of stay was affected.
CONCLUSION
Although it is acknowledged that there are significant limitations to a retrospective data into a restricted population treated with UDCA, there is potential that it could be protective if patients are strongly adherent.







