Authors: Nicola Humphry
Affiliation: Nottingham, UK
Disclosure: Humphry has no relevant competing interests to declare.
Acknowledgements: Writing assistance was provided by Nicola Humphry, Nottingham, UK.
Support: The expert talks and the publication of this article were funded by Boston Scientific. The views and opinions expressed are exclusively those of the speakers. The content was reviewed by Boston Scientific for medical accuracy.
Citation:EMJ Int Cardiol. 2022;10[Suppl 1]:2-9.
Meeting Summary
Calcific lesions, if not adequately pre-dilated, can prevent the successful expansion of a stent during percutaneous coronary intervention (PCI). In this series of educational videos, experts explained the importance of treating calcium, and the role of intravascular imaging to improve procedural success. Presentations included the optimal use of intravascular ultrasound (IVUS), when and how to use techniques such as rotational atherectomy (RA), and how to approach challenging cases such as nodular calcified lesions or bifurcation lesions.The Importance of Optimal Calcium Treatment
Alaide Chieffo
Lesion preparation through calcific plaque modification is necessary in patients with heavily calcified coronary arteries. The aim of lesion preparation is to allow successful stent delivery and adequate stent expansion and to prevent stent thrombosis and restenosis.
Depending on the severity of the calcification, lesion preparation might involve balloon angioplasty (such as cutting or scoring balloons), lithotripsy devices, and either rotational or orbital atherectomy. Atherectomy is often the best course of treatment if the lesion is uncrossable with a balloon.
Clinical trials assessing RA prior to drug-eluting stent implantation have indicated that this procedure is associated with higher strategy success than stent implantation alone,1 or modified (scoring or cutting) balloon angioplasty,2 in cases of severe calcification. These trials also found that acute lumen gain was greatest when calcified lesions were prepared with RA or cutting or scoring balloons prior to stent implantation,1,2 and the use of such techniques did not affect the incidence of clinical events such as in-stent restenosis and major adverse cardiac events.1,2 A strategy of pre-dilatation with a modified balloon prior to stent implantation failed in almost 20% of procedures, and successful completion required bailout atherectomy.2
More recently, the ISAR-CALC trial randomised patients with severely calcified coronary lesions, for whom lesion preparation with a standard non-compliant balloon was unsuccessful, to super-high-pressure balloon or scoring balloon preparation techniques.3 Intravascular imaging after drug-eluting stent implantation showed that both super-high-pressure balloon and scoring balloon techniques were associated with high levels of strategy success (92% versus 84%, respectively; p=0.48) and procedural success (100% versus 89%, respectively;
p=0.12).3 When a balloon of any type is used for calcium modification, full expansion is a critical measure of success, since the goal is to witness fracture of the calcific plaque. Therefore, it is important for the interventionist to check the balloon expansion with fluoroscopy.
Complications from calcium modification procedures are rare, but it is important to be prepared for them. Intravascular imaging should be used to make sure that the correct balloon or atherectomy burr and stent sizes are used,
and covered stents should always be available during the procedure in case a perforation
should occur.
The Utility of Intravascular Assessment of Calcified Lesions
Andrew Ladwiniec, Gabriele Gasparini, Pierre Deharo, and Pablo Salinas
Intravascular imaging helps an interventionist
get the best out of a PCI procedure. The choice of optical coherence tomography (OCT) or IVUS as an imaging modality for a calcified lesion should partly depend on the experience of the interventionist. It is crucial that findings are correctly interpreted and acted upon, and Pablo Salinas, Servicio de Cardiología, Hospital Clínico San Carlos, Madrid, Spain, explained that most of the measurements required to make clinical decisions for calcified lesions can be made with both OCT and IVUS. In the ‘School of Rock’ series of webinars, Andrew Ladwiniec, Department of Cardiovascular Sciences, University Hospitals of Leicester NHS Trust, UK; Gabriele Gasparini, Humanitas Research Hospital, Milan Italy; Pierre Deharo, Service de Cardiologie, Assistance Publique Hôpitaux de Marseille, France; and Salinas each discussed the use of IVUS for the assessment of calcified lesions.
IVUS imaging can be enormously supportive of a PCI procedure when calcification is present, particularly in patients with chronic kidney disease, where minimal use of contrast is needed. IVUS can differentiate a calcified plaque, which causes shadowing by reflecting the ultrasound signal from a fibrous plaque. It can, therefore, be used to assess lesions: pre-PCI, to determine if there is a need for calcium modification, and if so, to decide which tools to use; after calcium modification, to determine success; and post-PCI, to assess the success of the overall procedure (Table 1).
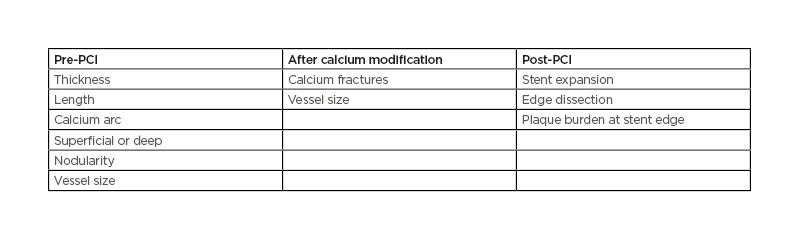
Table 1: A checklist for intravascular ultrasound assessment of calcific disease.
PCI: percutaneous coronary intervention.
Intravascular Ultrasound Assessment Prior to Lesion Preparation
With an initial pre-PCI IVUS, an interventionist should assess both vessel size, and the nature and distribution of calcium, measured by thickness, length, and calcium arc (Figure 1).4
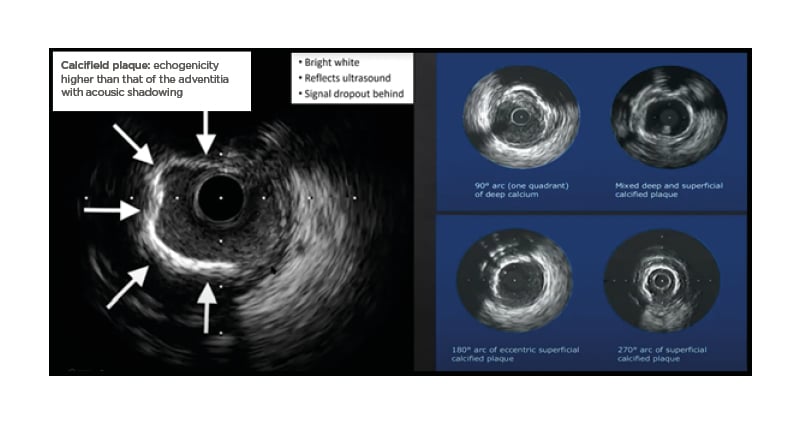
Figure 1: The identification of calcified plaque using intravascular ultrasound.
Reproduced with permission from Boston Scientific.4
IVUS is able to indicate whether calcification is superficial or deep. The degree of reverberation of the ultrasound signal can be used to predict the thickness of the plaque, as thick deposits block reverberation from the arterial wall behind the calcium. A smooth surface with areas of reverberation on IVUS is predictive of a calcium thickness of <0.5 mm on OCT, whereas a rough surface with no reverberations behind the
high-attenuation surface is predictive of thicker calcium (>1 mm).5 IVUS can also identify nodular calcified lesions, which appear as calcification with a convex luminal surface.
In a retrospective study, a calcified lesion thickness of >0.5 mm, a length of >5.0 mm, or a calcium arc of >180° (50% of the vessel circumference) were all predictive of stent under-expansion.6 Salinas calls this the “rule of fives;” if these features are present, calcium modification may be required for successful stent implantation.
Intravascular Ultrasound Assessment After Calcium Modification
Here, the interventionist should use IVUS to assess whether calcium modification has
been sufficient to permit full stent expansion. Markers of success include the introduction of fractures into the calcified lesion7 and/or a reduction in plaque thickness,6 depending on the techniques used.
If calcium modification was successful, the next step will be to select the most appropriate balloon and stent to use. However, if the calcium was insufficiently modified, further techniques will be required.
Intravascular Ultrasound Assessment After Percutaneous Coronary Intervention
IVUS should be used after a PCI procedure to assess the success of the stent implantation.
In the ULTIMATE trial, the IVUS criteria for optimal PCI were defined as a minimal stent area (MSA) >5 mm2 (or >90% of the distal reference segment); plaque burden of <50% within 5 mm of the proximal or distal stent edge; and no edge dissection involving media >3 mm. When all these criteria were met, the target vessel failure at 12 months was much lower than if they were not met (1.6% versus 4.4%, respectively; p=0.029).8
Stent expansion is the single most important factor related to long term outcomes;9 and Räber et al.,9 recommend similar targets to the ULTIMATE trial, suggesting that an interventionist should aim for an MSA >5.5 mm2 (or 7 mm2 in
the left main) by IVUS.
Using Intravascular Ultrasound to Guide the Choice of Calcium Modification Technique
IVUS findings should be used to guide the interventionist in terms of the best device to use, and many of the speakers in the School of Rock webinars offered their opinions and recommendations.
Ladwiniec stated his belief that deep calcification is generally more suited to balloon-based techniques such as lithotripsy or orbital atherectomy, whereas RA may be more appropriate for superficial calcium. Similarly, Deharo explained that he prefers to start with lithotripsy for deep calcium deposits, whereas he favours atherectomy in lesions with thick calcium or a length >10 mm.
Ladwiniec and Gasparini explained that thinner calcium may be easier to modify; if RA has successfully thinned the plaque, compliant balloon inflation before or after stent implantation may be sufficient for adequate stent expansion. Alternatively, RA can be followed by cutting balloon inflation to break up the calcium prior to stent implantation.
Ladwiniec has found lithotripsy to be particularly successful in cases with concentric calcification, whereas eccentric, nodular calcification is considered to be one of the greatest challenges in managing calcific disease. Options for the preparation of nodular calcified lesions are discussed later in this article.
Calcium Modification Techniques
Coronary interventionists have an array of techniques available to treat calcific coronary disease (Figure 2), yet Ladwiniec stresses that no single technology can address all calcification patterns. Many algorithms have been published to help the interventionist decide on the most appropriate technique in each situation; in all these algorithms, the use of intra-coronary imaging to guide correct treatment is key.
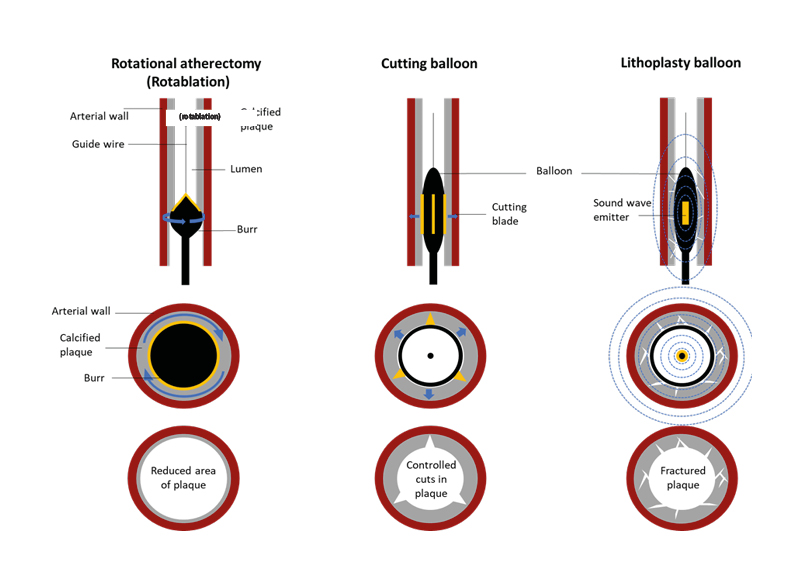
Figure 2: Tools available for calcium modification.
The first row of diagrams illustrates the entry of calcium modification tools into a longitudinal section of a coronary artery, while the second row represents the tools in situ in cross section. The third row of diagrams illustrates the effects of each tool on calcified plaque.
Indications and Technique for Rotational Atherectomy
Beatriz Vaquerizo and Anja Øksnes
The choice of RA as a calcium modification technique was discussed by Beatriz Vaquerizo, Department of Cardiology, Parc de Salut Mar, Barcelona, Spain, and Anja Øksnes, Department of Heart Disease, Haukeland University Hospital, Bergen, Norway, in two of the School of Rock webinars. Videos describing other techniques are also available on the School of Rock website.10
RA mainly effects the intima, resulting in a gain in the minimal lumen diameter (MLD [Figure 3]).11 It uses a differential cutting technique; normal, elastic vascular tissue deflects the advancing diamonds on the rotating burr, whereas plaque is inelastic, and will be selectively ablated.
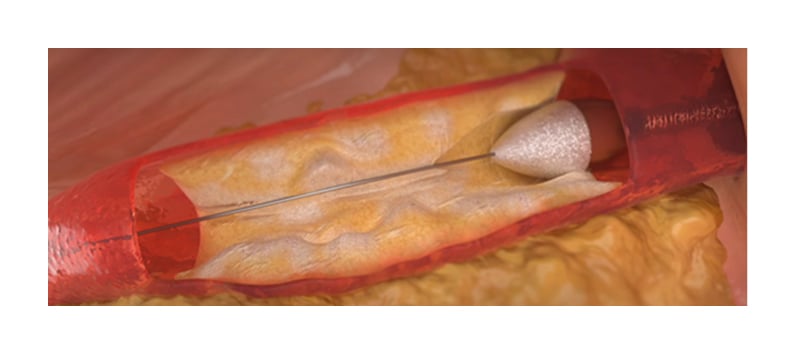
Figure 3: Illustration of a rotational atherectomy burr entering a region of calcified plaque in a coronary artery.
Reproduced with permission from Boston Scientific.11
Øksnes suggested that using RA to induce calcium fracturing to increase arterial compliance; to perform nodular debulking to achieve luminal gain; to facilitate safe equipment delivery; to reduce vessel failure12 by achieving adequate stent expansion and minimising eccentricity or asymmetry; to reduce procedural complications; and to increase procedural efficiency.
Vaquerizo explained that she is most likely to use RA in cases of small MLD, long lesions, uncrossable lesions, and undilatable lesions. For lesions with apparently mild calcification, Vaquerizo first tries to cross the lesion with a small diameter balloon. If the balloon cannot pass through the affected vessel, she will proceed to RA. On the other hand, if calcification appears to be moderate-to-severe, she tends to use intravascular imaging to get a clearer picture of the calcification pattern. Vaquerizo then attempts to use a cutting balloon for moderately calcified lesions, proceeding to RA if the balloon fails to cross the lesion, or cannot be fully expanded. She uses RA as
first-line treatment for severely calcified lesions.
Like Vaquerizo, Øksnes considers RA to be most useful in cases with moderate-to-severe calcification, balloon uncrossable lesions, or long calcified segments, although she feels the applicability of this technique in bifurcation lesions is less clear. Both Øksnes and Vaquerizo would consider RA for nodular calcium.
Vaquerizo is less likely to use RA in cases where substantial tortuosity or eccentric calcification is present; in proximal segments with a greater MLD, or in cases of in-stent restenosis, she prefers to use orbitational atherectomy or lithotripsy. Øksnes finds RA to be less effective against deep calcium lesions, in vessels with extreme angulation or tortuosity, or in bifurcations with a high risk of an important side branch.
There is little consensus on what constitutes optimal technique in RA, and opinions can differ between medical groups and countries. However, it is generally agreed that RA should start with a small burr with a burr-to-artery ratio of 0.5–0.6 (1.25–1.5 mm in most cases), and a rotational speed of 150,000–170,000 rpm. In Vaquerizo’s experience, higher speeds are better with smaller burrs, and lower speeds with larger burrs. Both Vaquerizo and Øksnes advocate gradual burr advancement using a pecking motion, which allows the operator to feel feedback through the guide wire. Decelerations >5,000 rpm should be avoided. Vaquerizo often follows rotablation with a cutting balloon to optimise calcium modification, though she is aware of other medical groups that prefer to use RA alone, with a gradual increase in burr size.
Most complications can be avoided with good RA technique.13 For example, slow flow can be avoided with the use of small burrs and lower speeds, and dissection can be minimised by avoiding cases with excessive vessel tortuosity. Burr entrapment is rare, and it can often be managed with a controlled push/pull of the RA shaft, or the positioning of a second guide wire to allow balloon placement. Perforation is commonly related to poor technique, such as the use of oversized burrs, excessive angulation, or inappropriate speed. If perforation does occur, it can be resolved with emergency pericardiocentesis and covered stents.13
The Challenge of Nodular Calcification
Andrew Ladwiniec and Gabriele Gasparini
Nodular calcification appears as a convex
luminal calcium surface using IVUS. It is considered one of the greatest challenges in managing calcific disease. Balloon-based techniques and lithotripsy are unlikely to be sufficient to modify these nodules; Gasparini explained that some of the lithotripsy will be reflected from the sides of the hard tissue nodule, resulting in less impact on the calcified plaque. Rotational or orbital atherectomy are likely to be required in these cases.
Nodules can create a wire bias, whereby the guide wire for the debulking technology is positioned closer to the soft tissue of the arterial wall than to the nodule. The position of an IVUS catheter can indicate the likelihood of bias, and providing that the bias is suitable, RA can be successful in removing nodular calcium deposits. If the interventionist is unable to ablate the nodule, subsequent stent implantation is likely to be affected by eccentric expansion and,
potentially, malapposition.
Calcium Modification in Bifurcation Lesions
Claudia Cosgrove
Claudia Cosgrove, St George’s University Hospitals NHS Foundation Trust, London, UK, explained that the combination of a bifurcation with calcification can be very challenging. As with any complex lesion, procedural decisions need to consider the patient characteristics (age, comorbidities, and left ventricular function); the anatomy of the lesion (substrate for ischaemia, bifurcation angle, calcium distribution); and the vessel size mismatch. The risk of complications from aggressive decalcification such as perforation, flow-limiting dissection, and ischaemia should be taken into account.
In bifurcations, plaque generally accumulates in the outer walls, promoted by low shear stress and increased blood turbulence; the carina is typically spared. Intravascular imaging, often after pre-dilation of the region with a semi-compliant balloon, helps to clarify the distribution of the calcium beyond the capacity of an angiogram. This can inform the strategy for calcium modification, such as ablation for bulky or nodular calcium deposits, and balloon-based techniques for concentric calcium. Imaging also helps to accurately assess the plaque burden at the ostia.
As with nodular calcification, wire bias can limit RA in bifurcations, and this potential issue can be detected through imaging, since the IVUS probe will also be affected by the same bias. The risk of perforation is higher in bifurcations than in linear vessels and is further increased when large angles or tortuous segments are present.
Balloon-based techniques can be associated with a risk of significant dissection extending down important side branches in bifurcations, creating flow limitations. In bifurcations that subtend a large amount of myocardium, the occlusion of an entire tree with a balloon can also cause significant ischaemia, and this risk can be mitigated with mechanical circulatory support. Side branch compromise can occur if plaque is pushed from the main vessel into the opening of a smaller vessel. Subgroup analysis of the PREPARE-CALC study showed that side branch compromise in bifurcations was more common with cutting/scoring balloon techniques than with RA (32% versus 7%, respectively; p=0.001), mainly due to the increased occurrence of residual stenosis in the side branch.14
Live Case Presentations
Several live calcium modification procedures were presented as part of the School of Rock 2021 webinars, each demonstrating the use of cutting-edge techniques across Europe.
Three cases demonstrated the use of RA. The first was a patient with impaired renal function, where IVUS revealed short, functional,
aorto-ostial occlusion with heavy calcification, plus collaterals and competitive flow in the distal right coronary artery (RCA) from the left system. The second involved RA of uncrossable RCA lesions and a tortuous distal left main, and the third was a case of significant RCA calcification, with likely disease extending back to the ostium.
One case demonstrated the use of RA followed by cutting balloon. This patient had multiple cardiovascular risk factors and pre-existing overlapping drug-eluting stents. IVUS revealed largely eccentric calcium, with some nodular disease, and IVUS was also used to select the landing zones for new stents.
The fifth case demonstrated a cutting-balloon procedure with the use of mechanical circulatory support in a patient with substantial comorbidity, including a porcelain aorta and previous radiotherapy to the chest. IVUS revealed thick calcification of a bifurcation lesion and a nodule in the distal left main, substantial occlusion of the ostium, and eccentric disease in the circumflex.
Take Home Messages
Several key points were made across the School of Rock 2021 webinars, which aimed to help coronary interventionists to understand when calcium modification is needed prior to PCI, which cutting-edge tools to use in different situations, and how to use RA with optimal technique:
Intravascular imaging is key to the appropriate use of calcium modification techniques and achieving good stent expansion.
Integrating IVUS to the complex PCI workflow improves results and saves both time and contrast medium.
Ideally, IVUS should be used pre-, peri-, and post-PCI to select the most appropriate calcium modification tool and assess its effect.
RA can facilitate procedural success, enabling the treatment of otherwise uncrossable or undilatable lesions.
Most potential complications are predictable and are technique-dependent.
In addition to the webinars reviewed here, the School of Rock also includes over 30 short, on‑demand, educational videos from experts throughout the world, to help interventionists improve outcomes in coronary calcified lesions. Topics include IVUS assessment, RA, cutting balloons, and combining RA with cutting balloons (the Rota-Cut technique).10








