Abstract
Aortic valve replacement is the mainstay of treatment for symptomatic severe aortic stenosis. In this setting, the rapidly evolving field of transcatheter aortic valve implantation (TAVI) is currently considered a safe alternative to surgical aortic valve replacement in patients with severe aortic stenosis who are considered inoperable or at high surgical risk. This review will focus on recent changes in the field of TAVI, describing patient selection, valve types, procedural approaches, short and long-term outcomes, and complications. The rapid evolution of TAVI procedures supported by solid evidence will, in the near future, probably extend the indications to a wider portion of patients with aortic stenosis.
AORTIC VALVE STENOSIS: SCOPE OF THE PROBLEM
Aortic valve stenosis (AS) is the most frequent degenerative heart valve disease in the Western world; it affects up to 2% of the population >65 years of age and its prevalence increases proportionally with age.1 The pathophysiology of AS includes processes similar to those of atherosclerosis, including lipid accumulation, inflammation, and calcification.2 Causes of AS are degenerative calcification (senile AS), congenital valve defects such as bicuspid valve, and rheumatic disease.3 While the development of symptoms (angina, syncope, or dyspnoea) demarks an inflection point in the survival of the patients with AS, the correlation between severity of the AS and onset of symptoms is poor and depends largely on the hypertrophic response of the left ventricle to the pressure overload.4,5 However, following the onset of symptoms, patients without aortic valve replacement have a poor prognosis with a median survival of around 2 years.4 Severe AS constitutes a growing and major health problem in developed countries, as it is the most prevalent cardiovascular condition after hypertension and coronary artery disease (CAD).5
TREATMENT STRATEGIES FOR AORTIC STENOSIS
In symptomatic patients with severe AS, the gold standard treatment was once a promptly performed surgical aortic valve replacement (SAVR). SAVR reduces symptoms and improves survival with low operative mortality in patients who are not at high surgical risk.6-9 However, one-third of patients with severe symptomatic AS would not undergo surgery due to advanced age, left ventricular dysfunction, or the presence of multiple co-existing conditions (pulmonary hypertension, porcelain aorta, etc.).4,10-12 Balloon aortic valve valvuloplasty procedures were considered as an alternative to SAVR, however their popularity declined due to poor long-term results;13 the transcatheter aortic valve implantation (TAVI) was subsequently developed in the 2000s.
In asymptomatic patients with AS, the management and clinical decision making are challenging. Symptomatic status can be difficult to establish, especially in elderly patients, who may ignore their symptoms or may reduce their level of physical activity to avoid or minimise symptoms. Exercise testing, such as exercise stress echocardiography, could thus be useful to unmask symptoms in patients with severe AS who claim to be asymptomatic or who have equivocal symptoms. Exercise testing is strongly advocated in the European Society of Cardiology (ESC) guidelines,5 whereas it is a Class IIb recommendation in the American College of Cardiology (ACC)/American Heart Association (AHA) guidelines.21
WHAT IS TRANSCATHETER AORTIC VALVE IMPLANTATION?
Alain Cribier performed the first TAVI14 in humans in 2002 and since then there has been an incredible growth of this technique, supported by a substantial amount of research.15,16 Following the Placement of Aortic Transcatheter Valves (PARTNER),17,18 CoreValve Pivotal,19,20 and NOTION21 trials, TAVI was included in the ESC/European Association for Cardio-Thoracic Surgery (EACTS) and the ACC/AHA Task Force on Practice Guidelines in 2012 and 2014.22,23 Current indications include inoperable and high-risk patients. In the latter group, the decision of TAVI versus SAVR should be made by multidisciplinary consensus within the Heart Team on a case-by-case basis. Expanded indications for TAVI (~10% of current procedures) include valve-in-valve procedures for degenerated bioprosthesis, AS due to bicuspid aortic valve, and pure aortic regurgitation, and the promising field of transcatheter intervention for pulmonary, tricuspid, or mitral valve disease.24 Mitral valve-in-valve and valve-in-ring implantations have been shown to be effective at treating failed mitral annuloplasty or bioprosthesis, and are associated with low rates of complications.25,26
DIFFERENT TRANSCATHETER AORTIC VALVE IMPLANTATION DEVICES
A complete description of every device is beyond the scope of this paper, but the market is still dominated by the Edwards SAPIEN (Edwards Lifesciences, Inc., Irvine, CA) and Medtronic CoreValve® (Medtronic, Minneapolis, MN) valves, with increasing use of DirectFlow, Boston Lotus, and St Jude Portico devices. The latest valve iterations are the SAPIEN 3, a balloon-expandable frame housing a pericardial tissue valve27,28 with a new outer polyethylene terephthalate cuff (to enhance paravalvular sealing) with the Commander transfemoral delivery sheaths of 14 Fr and 16 Fr; and the CoreValve Evolut™ R, resheathable, and repositionable self-expanding valve with a new 14 Fr delivery sheath.29 Important data derived from one randomised controlled trial and five observational studies compared these different devices and demonstrated, at 30-day follow-up, that rates of death did not differ between self-expanding and balloon-expandable valves, and rates of all-cause death did not differ at 1-year follow-up.30 In the 1-year results of the CHOICE trial comparing both valves, no differences in 1-year mortality rates were observed, despite the higher device success and lower paravalvular regurgitation (PVR) rate (which remained stable during follow-up) achieved with the Edwards valve.31
PATIENT SELECTION
Correct patient selection is crucial in order to achieve optimal results. Important points that should be considered include careful echocardiographic evaluation of left ventricular function, valve anatomy, other concomitant valve diseases, and confirmation of the severity of the AS. In patients with low-flow low-gradient AS, it is recommended to perform a stress echocardiograph, with exercise or dobutamine infusion. A cardiac computed tomography (CT) scan is now the most important imaging tool to study the aortic root and decide on the size of device to use. Issues with prognostic and functional significance are the load of valve calcification, size of aortic annulus and left ventricular outflow tract, morphology and calcification of aortic root, and location of coronary ostia.32
Surgical risk scores fail to accurately predict mortality after TAVI,33 however risk can be estimated using the Society of Thoracic Surgeons’ (STS) score (moderate risk 4–8; high risk ≥8–10 of predicted mortality), the Euroscore II, and the Logistic Euroscore. Current practice guidelines propose a careful evaluation of other risk factors not well reflected in those scoring systems, including frailty, poor mobility, obesity, end-stage liver disease, previous chest wall radiation, or cognitive impairment.34 Potential poor functional outcome in spite of a successful TAVI procedure should also be taken into account.35 The concept of medical futility has arisen in the TAVI scenario, and no invasive treatment should be considered if life expectancy with successful operation is <1 year; chance of death or major morbidity (all-cause) is high; or other major organ systems (≥2) are compromised with improvement not expected after operation.23,34
Once superiority of TAVI over medical treatment in inoperable patients and the equipoise to SAVR in high-risk patients was widely accepted, the research focussed on intermediate-risk patients. Early results from registries and the all-comers randomised NOTION trial are promising. In this trial, TAVI was safe and effective, and comparable to SAVR in regards to the composite rate of death from any cause, stroke, or myocardial infarction after 2 years.36 Recent data from the PARTNER 2 trial, which included patients at intermediate risk (mainly STS score <≥4) showed non-inferiority of TAVI compared to the SAVR in the primary outcome of death from any cause or disabling stroke at 2 years (19.3% TAVI versus 21.1% SAVR; p=0.25). Moreover, the TAVI group showed lower valve gradients and lower risk of bleeding events, acute kidney injury, and new-onset atrial fibrillation, as well as more rapid early recovery that resulted in shorter durations of stay in the intensive care unit and hospital.37
PROCEDURAL APPROACH
The current standard approach is the retrograde transfemoral, even though the first TAVI procedures were performed anterogradely through the atrial septum.14,38 Other approaches (transapical, subclavian, or transaortic) are indicated for patients with inadequate lower limb arterial tree (roughly one-third of TAVI patients).39-41 Selection of the best TAVI approach should be made on a case-by-case basis, focussing on the patient’s anatomy and local experience. Transfemoral access is generally associated with better outcomes and should be used, if feasible.42 Vascular access closure is key to avoiding bleeding and is usually obtained with percutaneous closure devices. The two most commonly used closure devices (Prostar and Proglide) were recently compared in an observational study that showed higher rates of major vascular complications in the Prostar group that contributed to a higher incidence of bleeding events and peri-procedural acute kidney injury, with no difference in mortality.43
CAD is a common comorbidity in the TAVI population, present or detected during pre-procedural coronary catheterisation in approximately 60% of TAVI candidates.44 Even if there is no established strategy of how and when to treat CAD, European guidelines for myocardial revascularisation provide an IIa–c recommendation for percutaneous intervention of stenosis >70% in proximal coronary segments in patients undergoing TAVI.45 Nevertheless, conflicting results exist concerning the influence of CAD on outcomes, and controversy persists regarding the extent and timing of revascularisation prior to a TAVI procedure.46
Another interesting field of investigation is the conventional versus minimalist approach. General anaesthesia and transoesophageal echocardiography guidance is being challenged by conscious sedation and local anaesthesia. The 3M TAVI study evaluated the efficacy, feasibility, and safety of next day hospital discharge in TAVI utilising the Multidisciplinary, Multimodality, but Minimalist (3M) approach compared with standard TAVI management (usually a 3–5 day stay after procedure). Preliminary data showed a feasibility of next day discharge in 97% of enrolled patients with only 3% re-admissions within 30 days.47 The minimalist approach could also reduce the cost of the procedure. In a study that compared in-hospital cost between TAVI and SAVR, TAVI was demonstrated as an economically satisfactory alternative to SAVR, with a ~2-day shorter length of stay.48 However, the most promising prospect regarding cost of the procedure is the expected reduction in valve device prices.49
PROCEDURAL OUTCOMES
The efficacy of TAVI in the treatment of patients with AS has been demonstrated in robust registries and large-scale studies.17-20,21 The short-term outcomes from recent registries are summarised in Table 1. Indeed, a large meta-analysis of patients undergoing TAVI identified high pro-b-type natriuretic peptide levels and post-procedural acute kidney injury as the strongest independent predictors of 30-day and 1-year mortality.50 In patients with mitral regurgitation (MR), a recent meta-analysis associated a concomitant moderate-severe MR with an increase in early and late mortality following TAVI. Half of the patients had a significant improvement in MR severity (greater in those who had received a balloon expandable valve).51 There has been a gradual improvement in outcomes (increased success and reduced complication rates), probably due to the technological advances and the increased experience of the operators.
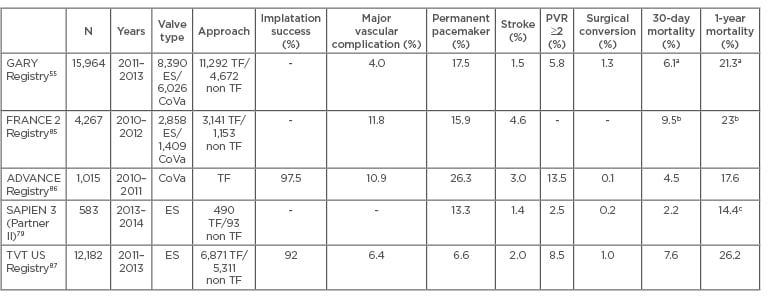
Table 1: Short and mid-term results of the main multicentre registries and controlled trials.
a) Follow-up data from 9,091 patients (2011–2012). 30-Day mortality was 5.4% (TF) and 8% (TA); 1-year mortality was 19.8% (TF) and 24.9% (TA) (TF-TAVI versus TA-TAVI: p<0.001), b) Follow-up data from 3,195 patients, c) 1-year survival: 85.6% overall, 87.3% high-risk patients, and 89.3% high-risk TF access.
ES: Edwards-SAPIEN; CoVa: CoreValve; TF: transfemoral; TA: transapical; TAVI: transcatheter aortic valve implantation; PVR: paravalvular regurgitation.
A few studies have follow-up data available for up to 4–5 years, all of them with a high mortality rate (>50%) at 5 years. It is important to emphasise that these studies included a very old population with significant comorbidities (high or extreme risk), some of them could probably be excluded from a TAVI procedure nowadays due to excessive risk (futility). The 5-year follow-up of the PARTNER trials published equivalent outcomes for high-risk patients who underwent SAVR or TAVI: there were no significant differences in all-cause mortality, cardiovascular mortality, stroke, or need for re-admission to the hospital. In addition, the functional outcomes were similar, and no differences were demonstrated between surgical or transcatheter valve performance. Other observational studies confirm similar results with 5-year mortality rates of 61–68% (Table 2).
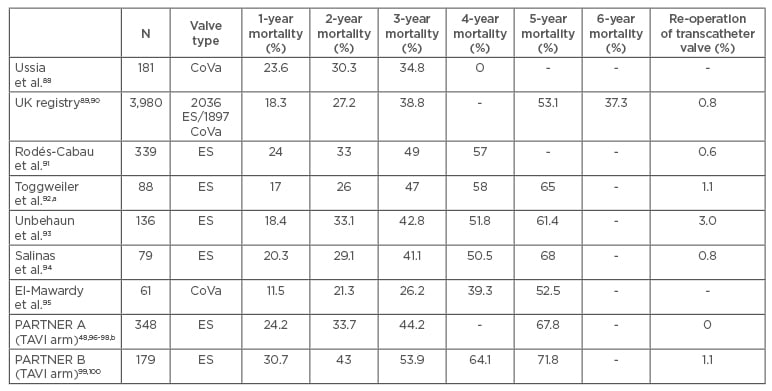
Table 2: Long-term mortality results after transcatheter aortic valve implantation. Procedures performed between 2007 and 2012.
a) Selected population, excluding implantation failure or death before 30 days. Follow-up in 84 out of 88 patients, b) re-operation date reported at 2 years of follow-up.
ES: Edwards-SAPIEN; CoVa: CoreValve; TAVI: transcatheter aortic valve implantation.
COMPLICATIONS
With some disparity in the initial TAVR reports, the Valve Academic Research Consortium (VARC) one52 and two34 criteria helped to standardise reporting of postoperative complications. An outline of the most frequent complications or novel findings will be addressed in this section, although many other complications may occur during or after a TAVI procedure.
Bleeding
More than two-thirds of patients are now undergoing TAVI through the transfemoral route with a percutaneous arterial closure device.53 The transfemoral approach is associated with higher vascular complications compared to the transapical TAVI,54 however these have been significantly reduced by the introduction of the current reduced sized sheaths.55 Hitherto, bleeding was one of the most relevant complications of transfemoral TAVI. Although VARC consensus documents recommend the Bleeding Academic Research Consortium (BARC) criteria, peri- procedural and long-term bleeding is inconsistently reported. There are also difficulties in quantifying procedural blood loss and in the reporting of the precise reasons for peri-procedural transfusion.
In the first TAVI devices 30-day bleeding could be as high as 41% (15.6% life-threatening), and the rate of transfusions reached 43%.56 However, data from the PARTNER trial shows that TAVI considerably reduces bleeding and transfusions compared to SAVR.57 Improvements in delivery catheters and experience cut current procedural bleeding in half (down to 6.3% life-threatening).58 Patients who require a blood transfusion following TAVI exhibit an increased risk of major stroke and kidney dysfunction, as well as increased mortality at 1 year.59 Risk factors for life-threatening bleeding following TAVI include female gender, using a larger size delivery system (>19 Fr), peripheral arterial disease, valve retrieval, and percutaneous access.60 Late (>30 days) bleeding complications are low (5.9%), occur mainly (64.1%) in the first 6 months post-TAVI, and are associated with 1-year mortality, especially in atrial fibrillation patients.61 Current guidelines recommend double antiplatelet therapy after TAVI to prevent thromboembolic events, which could be associated with a higher rate of major bleeding. The POPular TAVI trial is the first large, randomised, controlled trial to test if monotherapy with aspirin or oral anticoagulation versus additional clopidogrel after TAVI reduces bleeding (enrolment ends in August 2016).62
Conduction Disturbances
The incidence of new onset left bundle branch block (BBB) varies with valve system and time after TAVI (10–50%), but most of them are transient and may be resolved by discharge in 30–50%. A recent study described that conduction disturbances occurred primarily during hospitalisation (increase of PQ interval, QRS width, and first grade atrioventricular block) and subsequently stabilise during a 1-year follow-up. Post-procedural complete left BBB was more frequent in the CoreValve and transapical approach.63
Inconsistent data have been published on whether left BBB after TAVI increases the risk of mortality. Complete atrioventricular block requiring a permanent pacemaker implantation (PPI) varies widely among studies and devices, and may be 5–12% for SAPIEN and 24–33% for CoreValve.64 This conduction disturbance is caused by damage to the atrioventricular bundle or node,65 and is often peri-procedural but may be delayed up to 7 days after the procedure. The need for PPI is higher with CoreValve and Lotus implantation compared with the SAPIEN device. A pre-existing right BBB and deep valve implantation are the main predictors of subsequent PPI.66,67 Although a new PPI could be associated to a decrease or lack of improvement in left ventricular function or increased rehospitalisation, it has not been linked to increased mortality.68
Stroke
The occurrence of cerebrovascular events is one of the most fearsome complications of TAVI. According to VARC definitions, procedural stroke (acute, <24 hours) has an incidence of 1.5% after TAVI. During the first month after TAVI (subacute) stroke has an incidence rate of 3–7%, reducing to 2.3% >1 year TAVI (late).69,70 Imaging studies following TAVI showed new embolic events in 72.0%; however, only 6.6% of those patients presented with clinically significant neurological deficits.71 The clinical relevance of this form of silent cerebral ischaemia remains to be resolved, though it has been speculated that it could be associated with a higher rate of cognitive decline in comparison with SAVR. The stroke rate has already declined with new-generation devices,72,73 and a great effort is being made to assess the added value of embolic protection devices (filters or debris deflectors) during TAVI procedures.
Paravalvular Regurgitation
An inadequate sealing between the prosthesis and the annulus with the crushed native leaflets causes this complication. Severe and/or asymmetrical calcification of the annulus, the presence of a bicuspid aortic valve, and inadequate prosthesis sizing are risk factors for PVR after TAVI. An exhaustive preoperative CT assessment of valve calcification and anatomy of the aortic root complex should minimise the risk of PVR.74 Approximately 70% of patients after TAVI have mild regurgitation,75 and the incidence of moderate or severe PVR is 15–20%.76 The quantification of the aortic regurgitation after TAVI can be done by angiography, by echocardiographic evaluation, or by invasive haemodynamic parameters.77 However, the assessment and grading of PVR has become a challenge due to disagreement between techniques, methodologies, and even core laboratories.78
PVR has consistently been associated with increased long-term mortality, although some conflicting data persist on the true impact of PVR due to grading assessment, different study populations, and device differences (PVR could be higher at post-implantation but reduce over time with CoreValve).31
Potential treatments of this complication are post-dilatation, second valve implantation, or selective leak closure with a vascular plug. However, any post-implantation optimisation procedure might convey a risk of embolisation or root injury, so new iterations of the valve devices brought specific designs to prevent PVR, with promising initial results.79 Some authors suggested prosthesis oversizing to improve adaptation to the aortic annulus, and thus minimise the leakage,80 although some other reports show conflicting results.81 In summary, the PVR is a multifactorial issue and warrants careful pre-operative evaluation, individualised implantation parameters (sizing, balloon volume), and expert management should the complication occur.
Valve Thrombosis
Probably the most commented on TAVI controversy in 2015 was the novel finding of subclinical leaflet thrombosis in bioprosthetic heart valves. Originally suggested in a CT evaluation at 30 days in the Portico IDE Study, and gathering the evidence from other studies, incidence varies from 7.4–43.2% across different devices (including surgical bioprostheses) and timepoints.82 The finding was consistently missed by transthoracic echocardiography but could be detected by transoesophageal echocardiography, and was resolved in all cases following warfarin therapy. There are still insufficient data to link this finding to clinical events.
Likewise, a TAVI thrombosis registry gathered retrospective data on clinically relevant (dyspnoea or increased valve gradients over time) valve thrombosis, usually detected with echocardiography. Incidence of valve thrombosis was 0.61% (possibly underreported due to the retrospective design), appearing up to 2 years after TAVR, and resolved in most cases with warfarin.83
OPEN ISSUES AND FUTURE DIRECTIONS
Cumulative evidence has demonstrated the value of TAVI in the treatment of patients with severe symptomatic AS for inoperable or high-risk patients. Moreover, data from a recent randomised controlled trial suggest that TAVI might be superior to SAVR in this setting.19 Expanded indications for intermediate-risk patients will follow should the upcoming PARTNER 272 and SURTAVI84 trials show positive results. Furthermore, a trial on low-risk patients (STS score <4) has been recently approved (PARTNER 3) and will have started in spring 2016. Durability >5–10 years will become highly relevant if TAVI is to be offered to low-risk patients with higher life expectancy.
New TAVI devices, most of them with self- expanding, recapturable, and repositionable technology, are being developed to further optimise results and reduce complications. Competition between devices will follow so implantation success, complications rates, long-term durability, price, and reimbursement systems will be important differentiating factors. Procedural simplification with a minimalist approach and advanced imaging modalities will increase TAVI application and its implementation in the treatment of other heart valve diseases. Off-label indications for valve-in-valve procedures similar to TAVI, implantation in different valvular heart diseases (aortic regurgitation, mitral, or tricuspid valve disease), or bicuspid aortic disease will expand; and more importantly, the technique will be thoroughly researched, hopefully with randomised, specific trials.24
Antithrombotic treatment after TAVI is one of the most important gaps in evidence at this moment, with a lack of support for specific regimes. A balance between recognised problems at both ends of the spectrum (late bleeding and subclinical valve thrombosis) must be found, and tailored treatments for specific populations given accordingly. The selective or widespread role of embolic protection devices for stroke prevention will be clarified with upcoming trials.
In spite of the increasing numbers of TAVI procedures, registries show no decline of SAVR, clearly demonstrating how senile AS is an undertreated disease. Should intermediate-risk trials have positive results for TAVI, it could become a standard of care, followed by a possible reduction in SAVR numbers. Beyond technical (device and procedure) details, keeping a multidisciplinary perspective on candidate selection will ensure continued improvements in survival and quality of life for AS patients, warranting a bright future for TAVI.








