Abstract
In recent decades, tremendous medical advances have been made. Therapeutic cardiac catheterisation for repair of congenital heart defects has become the standard mode of therapy. Catheter techniques have progressed. They now provide temporary palliation, prepare the patient for surgical reconstruction, or offer a definitive repair. The main advantages of non-surgical procedures are avoidance of thoracotomy and cardiopulmonary bypass, together with a shorter hospitalisation period and speedier convalescence.
Paediatric interventions include: transcatheter device closure of congenital cardiac defects, balloon angioplasty and valvuloplasty, atrial septostomy, patent ductus arteriosus stenting in the neonatal period, vessel embolisation, and many others. Topping those interventions is the introduction of transcatheter valve replacement. The aim of this article is to review these interventions and present them in a simplified, vibrant, and up-to-date fashion.
In conclusion, paediatric cardiac interventions have established their reliability and ever-expanding scope in the setting of congenital heart disease management. Nevertheless, success is dependent on selecting the proper procedure for each condition, which may also vary with each patient. Thus, it is highly dependent on the experience and expertise of the operator. With the current rate of technological innovation, more and more surgical procedures will eventually be replaced by catheter-based interventions with a great degree of safety and efficacy.
INTRODUCTION
The last five decades have witnessed the most remarkable advances in the management of congenital heart defects (CHD). In the last two decades these advances have become so safe and effective that they are becoming an attractive alternative to surgery, sparing patients the surgical scars. The patient is usually mobile the very same day and thereafter; most of these patients remain unaffected for the rest of their lives.1 From the most common defect, atrial septal defect (ASD), to the rarest defect, aortico-ventricular tunnel, every unwanted defect in the cardiovascular system can be closed with various types of devices. As a result of the tremendous progress in the field of interventional cardiology, the scope for application of transcatheter procedures has widened.2
DEVICE CLOSURE OF ATRIAL SEPTAL DEFECTS
The ostium secundum type of ASD can be closed by the transcatheter technique. Different devices were used in the past.3 Dr Kurt Amplatz, former Professor of Radiology at the University of Minnesota, Minneapolis, Minnesota, USA, has developed the new self-centring device (Amplatzer™ septal occluder) to overcome the limitation of all earlier devices. It consists of a self-expandable double disc nitinol mesh. The device is securely screwed into a delivery cable and loaded into a long introducer sheath that passes from a femoral vein to cross the ASD. The distal disc is released in the left atrium, pulled against the septum, then the rear disc is released in the right atrium. When secured in place it is disconnected. The procedure is monitored by either transthoracic echo, transoesophageal echo (Figure 1A), or by intracardiac echocardiography.4 Recently, 3D reconstruction has helped tremendously in visualising the device after delivery and its relation to all surrounding structures (Figure 1C).
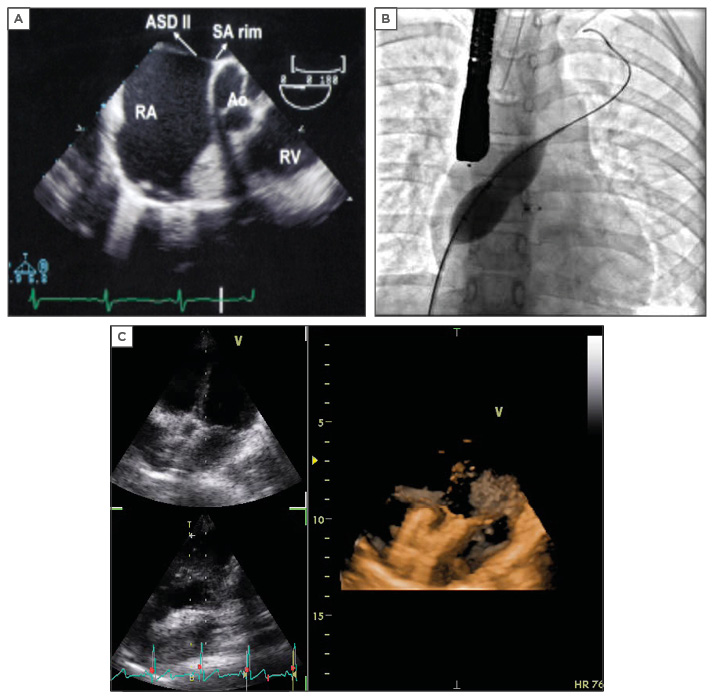
Figure 1: Imaging and device placement for an atrial septal defect.
A) Transoesophageal echocardiography showing ASD secundum; B) Balloon sizing as seen in catheterisation laboratory; C) 3D imaging showing an ASD device in situ with no residual shunt.
ASD: atrial septal defect; RV: right ventricle RA: right artery; SA rim: superior anterior rim; AO: aorta.
Some ASDs are considered difficult to work with, including those that are >26 mm. However, it should be noted that it is possible to close ASDs as large as 36 mm; balloon sizing is recommended in such cases (Figure 1B). Multiple ASDs can be closed as effectively with multiple devices, the smaller device sandwiched by the bigger device or large patent foramen ovale (PFO) device can cover all the multiple fenestrations.5 Symptomatic PFO patients with a history of cryptogenic stroke and PFO patients with a septal aneurysm can be closed with a PFO device.6 A septal aneurysm by itself is considered a challenge.
At least a 5 mm rim of atrial septum around the defect is needed as a prerequisite for device closure. A deficient or absent rim is anything <3 mm. However, the device design would not require the anterior rim for anchoring as it would splay around the posterior wall of the aorta.7 Some techniques were developed to aid the operator when attempting closure of defects with deficient rims, the most famous of which are the pulmonary vein approach, the Hausdorf sheath, and the balloon-assisted techniques.8-10
The other type of ASD devices are the patch devices, such as the Gore® Helex® Septal Occluder and some of the newer devices with biodegradable matrix that are being tried in animal models. The bioengineered collagen occluder is ideal for septal occlussion. The tissue scaffold promotes the healthiest and most complete healing response, excellent biocompatibility, and full resorption of material, leaving only native tissue.11
Atrial Septal Defect Closure in Special Situations: Infants, Elderly, and Pulmonary Atresia with Intact Ventricular Septum
Evidently, ASD must be closed in symptomatic infants with right ventricular volume overload, symptoms of pulmonary over-circulation, evidence of pulmonary hypertension, failure to thrive, or inability to wean off the ventilator. In this group of patients, surgical closure may pose some morbidity or even mortality. Therefore, efforts should be made to close them either percutaneously or utilising a hybrid approach, avoiding the complication of bypass and shortening the recovery period.12
An interatrial communication is usually present in cases of pulmonary atresia with intact interventricular septum for decompression of right chambers or increasing the left ventricular output. Ideally, atrial shunt closure should always be performed in these patients after they undergo right ventricular decompression if they have mild-to-moderate systemic desaturation (>85%) with bidirectional atrial shunt. However, it is absolutely contraindicated to close this communication if the shunt is still exclusively right to left.13
DEVICE CLOSURE OF VENTRICULAR SEPTAL DEFECTS
Ventricular septal defects (VSD) are the second most common CHD, accounting for ~20% of cases. About 75% of them are perimembranous and the remaining are muscular VSDs (mVSDs). Surgical closure of mid-mVSD, especially multiple defects, has high incidence of recurrence and is associated with high risk of morbidity and mortality.1 Today, mid-mVSDs or apical VSDs can be safely closed with the mVSD occluder. This device has a retention skirt 8 mm larger than the central waist and the length of the waist is 7 mm to fit in the thick interventricular septum well. The device can be effectively positioned after securing an arteriovenous circuit. More recently, the sole use of the transvenous approach has been attempted. The relatively small delivery system (6–9 Fr) allows the device to be placed even in small infants.14
Patients with haemodynamically significant mVSDs may be offered percutaneous or hybrid-approach device closure.15 Periventricular device closure of mVSDs is effective and safe, which makes it the approach of choice for small infants.16 Multiple mVSDs are difficult to manage as they develop pulmonary hypertension early. Today these multiple VSDs are closed by multiple VSD devices.17
Perimembranous VSD closure is challenging because of the thin membranous septum and its proximity to both the aortic and tricuspid valves. There is a higher possibility that it will impinge on the aortic valve, causing aortic regurgitation or compression of the AV node and subsequently cause atrioventricular block. It has been shown recently that perimembranous VSDs can be closed successfully using several devices: the perimembranous VSD device,18 the Amplatzer™ Duct Occluder I19 (Figure 2A and Figure 2B) or even the Amplatzer™ Duct Occluder II20 (Figure 2C and Figure 2D), and lastly the PFM medical coil occluder.21
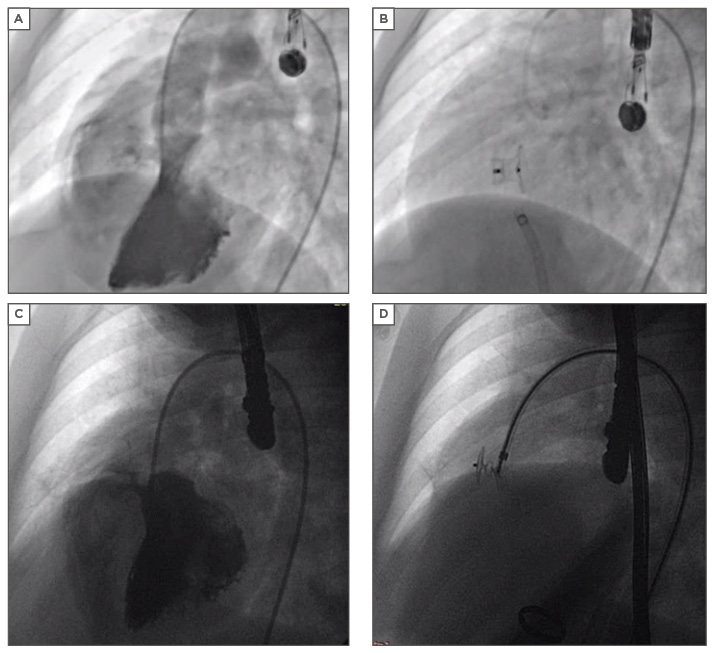
Figure 2: Device closure of a ventricular septal defect.
A) Moderate sized perimembranous VSD; B) VSD closed by ADO I; C) Small perimembranous VSD; D) VSD closed by ADO II.
VSD: ventricular septal defect; ADO: Amplatzer Duct Occluder.
DEVICE CLOSURE OF PATENT DUCTUS ARTERIOSUS
Percutaneous attempts to close the patent ductus arteriosus (PDA) started with the Ivalon plug in 1971. This prototype led to the development of a number of devices, like the Sideris button device and Rashkind’s umbrella devices. Nevertheless, these devices needed relatively large delivery systems. Then coils with thrombogenic strands, which can be delivered at the PDA through 5–6 Fr catheters became available. The success of these simple, safe, and cheap coils has opened the doors for newer detachable coils with smaller delivery systems using screws, which offered safe and controlled coil detachment. These coils can be relocated or withdrawn during the procedure if they are not in optimum position.22 The development of the Amplatzer Duct Occluder made it safe and easy to close the larger ducts.
Indications for duct occlusion are mainly to prevent the development of pulmonary vascular disease and to eliminate the possibility of infective endocarditis. Today transcatheter closure of PDA has become the standard of care replacing surgery, except in very low birth weight patients or those with complex anatomy. The choice of the device to use during PDA closure depends mainly on the experience and preference of the operator, but the general consensus is:
a) Small PDA <3 mm are closed with Cook’s coils, which have thrombogenic strands and are cheap and effective. The coils can be delivered both antegradely and retrogradely. The success rate reported for coils is up to 95%23
b) For moderately large PDAs (3–6 mm) we now have the Nit-Occlud® PDA occlusion system (Figure 3A and Figure 3B) which consists of a spiral coil made of nitinol with graduated stiffness and compact winding with memory. It mechanically occludes the duct as there are no thrombogenic strands24
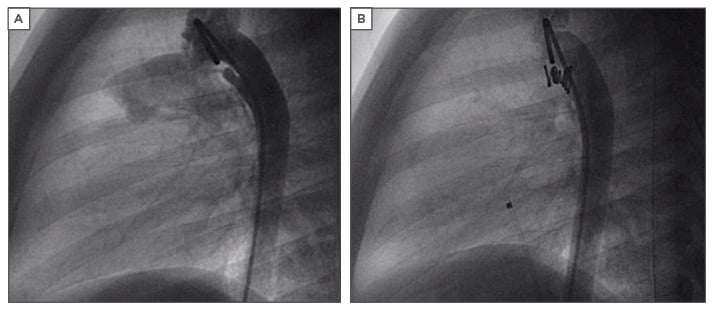
Figure 3: Device closure of a patent arteriosus ductus.
A) Elongated conical PDA on angiogram; B) Nit-Occlud PDA device in situ.
PDA: patent arteriosus ductus.
c) For larger PDAs (4–14 mm), Amplatzer Duct Occluder is available.25 The transvenous approach is used to deliver the aortic skirt in the ampulla
d) Recently, the Amplatzer Duct Occluder II and Amplatzer AS have been specially designed for infants, mostly <6 kg. This newer generation of devices are self-expanding with two retention discs (at both aortic and pulmonary ends) that articulate with a central plug. The advantage of these newer devices is that they have low profile and can be easily delivered through a 4 or 5 Fr sheath in infants. As these devices have a retention skirt on either side, they can be delivered either antegradely or retrogradely26
e) Closing tubular PDA in infants is still a very challenging task; Amplatzer™ Vascular Plug II and IV are safely used with 100% occlusion rates27,28
AORTOPULMONARY WINDOW
The aortopulmonary window is a communication between the ascending aorta and the pulmonary trunk and/or right pulmonary branch. It is rare, occurring in 0.2–0.6% of patients with CHD.29 In general, these malformations initiate significant left-right shunt with congestive heart failure in the first days or months of life, and early development of severe pulmonary hypertension.30 Several isolated reports were published showing the effective transcatheter closure of aortopulmonary window with different devices like the Amplatzer Septal Occluder and Amplatzer Duct Occluder II.31
CATHETER CLOSURE OF CORONARY ARTERY FISTULAE
Coronary artery fistulae are rare anomalies characterised by direct communication between one or more coronary arteries and a cardiac chamber or a great vessel bypassing the capillary network. Most fistulae arise from the right coronary artery and less frequently from the left coronary artery or the circumflex artery.32 Most cases are small and asymptomatic accordingly. Indications for closure are: heart failure, cardiomegaly or dilated left ventricle on echocardiography, aneurysmal fistula, coronary fistula in the setting of single coronary, and evidence of myocardial steal on stress electrocardiogram.33 Multislice computed tomography prior to procedure gives detailed anatomical information about the fistulae, drainage, and the narrowest site. This information helps in planning and preparation of the devices that are appropriate for the procedure.34 The device is chosen according to the size and shape of the fistulae, this can be coils intertwined together, vascular plugs, or nitinol occluder devices.
VESSEL EMBOLISATION OF PULMONARY ARTERIOVENOUS MALFORMATIONS, AORTOPULMONARY COLLATERAL ARTERIES, AND VENOVENOUS COLLATERALS
Pulmonary arteriovenous malformations are direct communications between the pulmonary arteries and veins bypassing the capillary bed.35 Embolisation is recommended in symptomatic patients or when the feeding arteries are >3 mm in diameter.36 Aortopulmonary collateral arteries can be detected in association with various CHD, especially complex cyanotic heart defects. In those patients, aortopulmonary collateral arteries can relieve systemic hypoxaemia prior to surgical correction. Surgical ligation is technically challenging. Indications of transcatheter embolisation are large left-right shunting, resulting in congestive heart failure, pulmonary over-circulation, respiratory compromise, and pleural effusion or protein- losing enteropathy.15
Venovenous collaterals are found most often in children with a univentricular heart after a bidirectional Glenn or modified Fontan procedure. The resulting increased systemic venous pressure after these palliations allows for flow through the pulmonary vasculature.37 Transcatheter embolisation is indicated if the collateral vessels are clinically relevant, causing significant cyanosis or increasing risk of systemic embolism.15 The embolising material for all these vessels includes coils, vascular plugs, and nitinol occluders.
Transcatheter Interruption of Antegrade Pulmonary Artery Flow in Status Post-Bidirectional Glenn
The key to a successful Fontan-type operation is low pulmonary artery pressure (PAP) and low pulmonary resistance. According to the classical indications for the Fontan operation, mean PAP should be <15 mmHg. Recent case reports have shown children with elevated mean PAP after a Glenn procedure, with additional antegrade pulmonary flow through the band, that could be treated successfully with transcatheter closure of this communication using occluder devices like ASD, VSD, or PDA.38
Transcatheter Completion of Fontan
Surgeons and interventionists have been collaborating to avoid repeated surgeries by developing new techniques that surgically precondition the heart prior to transcatheter completion. Special occluding stents have been reported to establish inferior vena cava: right atrium to pulmonary artery connection.39,40
Fontan Fenestration Closure
Lateral tunnel Fontan baffle fenestration and subsequent catheter closure was first described in 1990 to increase postoperative survival and decrease postoperative morbidity in patients at high-risk for transformation to this type of circulation.41 This results in decompression of systemic venous channel into the left atrium in cases with borderline Fontan physiology. Patients with chronic Fontan baffle leak with favourable dynamics and tolerating baffle occlusion testing can undergo transcatheter closure for the purpose of relieving cyanosis.15
LIFESAVING NEONATAL PALLIATIVE PROCEDURES
Transcatheter Balloon Atrial Septostomy
Enlarging of the ASD by rapidly pulling a balloon filled with radiopaque dye across the restrictive PFO was described by Rashkind and Miller in 1966.42 Since then thousands of neonates have been saved by this technique all over the world. Balloon atrial septostomy is a lifesaving procedure for severely hypoxic and acidotic neonates with congenital cyanotic heart diseases like transposition of great arteries, tricuspid, mitral atresia, and pulmonary atresia.2
Patent Ductus Arteriosus Stenting
This represents another neonatal lifesaving procedure described rather recently and replacing the need for quick surgical intervention in the neonatal period.43 The procedure allows the patient to stabilise and develop before returning after several months for a bilateral cavopulmonary (Glenn) shunt to be established and later completion of Fontan. The present coronary stents have better profile, flexibility, and tractability that can be delivered safely through a 4–5 Fr sheath. Tortuous PDA still represents a technical challenge.44
Ballooning/Stenting for Coarctation
Endovascular stents are used in conjunction with balloon dilatation to treat coarctation (native or post-surgical) and lately even in neonatal cases.45,46 Stents prevent recurrence of coarctation, recoil, and vascular proliferative responses. Stents can also prevent aortic dissection and aneurysm formation.
BALLOON DILATATION OF STENOTIC VALVES
Pulmonary Balloon Valvuloplasty
Kan et al.47 reported the technique of balloon dilatation of the stenotic valve with a significant reduction in the right ventricular pressure. Today, percutaneous balloon valvuloplasty is the treatment of choice for isolated pulmonary stenosis (PS) and neonates with critical PS. Pulmonary valvuloplasty is indicated for a patient with critical pulmonary valve stenosis (defined as PS present at birth with cyanosis and evidence of PDA dependency), at any age post-neonatal with or without right ventricle dysfunction and a peak-to-peak catheter gradient or echocardiographic peak instantaneous gradient of >40 mmHg, with the patient fully sedated.15
The balloon chosen should be from 1.2–1.6-times the pulmonary annulus measurement. High pressure balloons are chosen for dysplastic valves with tendency to recoil. The best results are obtained in doming valves with neither sub-valvular nor supra-valvular obstruction. Perforation of the pulmonary valve in the setting of pulmonary atresia/intact ventricular septum has made it possible to transform the pulmonary atresia to severe stenosis and thereafter proceeding with percutaneous balloon valvuloplasty.48
Balloon Valvuloplasty for Aortic Stenosis
Aortic valve balloon valvuloplasty is an effective and safe procedure for which the results are comparable to those of surgical aortic valvotomy. In neonates presenting with left ventricular failure, balloon valvuloplasty is lifesaving. It not only reduces the gradient across the aortic valve but also improves the left ventricular function and postpones the need for definite procedure-like valve replacement so that if required it can be replaced by a bigger adult size prosthetic valve or homograft.2,49
Indications for intervention include: a) critical aortic stenosis in the neonate with left ventricle dysfunction and PDA dependency; and b) aortic stenosis in childhood with peak systolic gradient >50 mmHg in symptomatic patients, patients with electrocardiogram changes, or peak systolic gradient >70 mmHg in asymptomatic patients with no electrocardiogram changes.50 The procedure is better done under pacing of the heart and the balloon annulus ratio should not exceed 0.9.
BALLOON/STENT ANGIOPLASTY FOR STENOSIS OF PULMONARY ARTERY BRANCHES
Peripheral pulmonary artery stenosis may be congenital or acquired (postoperative). Significant stenosis requiring intervention is when there are gradients of 20–30 mmHg across the stenotic area, when there is elevation of the right ventricle or proximal main PAP to greater than one-half of systemic pressure secondary to the distal obstruction, or when there is relative flow discrepancy between the lungs of 35/65% or worse.15 Surgical treatment of these stenoses is difficult and frustrating with very high incidence of recurrence. Recently, 3D reconstructed rotational angiography has facilitated balloon angioplasty with or without stenting, single or multiple, and has shown efficacy, becoming a new hope to these patients with peripheral pulmonary artery stenosis.51
TRANSCATHETER PULMONARY VALVE REPLACEMENT
Free pulmonary regurgitation may result in progressive right ventricle dilation and failure, arrhythmias, and progressive widening of the right ventricular outflow tract, leading eventually to death. Previously, these patients needed surgical valve implantation to preserve the ventricular function. Bonhoeffer et al.52 were the first to report on a novel percutaneous pulmonary valve technique. This technique is being refined regularly, aiming to use smaller sheaths and to ensure fewer complications like stent fracture, arrhythmia, homograft rupture, and coronary artery compression. An example of this is the newly introduced Venus P-Valve™ system.53
Currently, this procedure can only be done for children >5 years and weighing >25 kg, following multislice computed tomography and cardiac magnetic resonance imaging for reconstruction of the outflow tracts to aid accurate choice of the size to be used and the proper plane of valve deployment.
CONCLUSION
Transcatheter interventions have transformed the management of CHD. Device closure of various defects has provided spectacular improvement in patient care. Other interventions are increasing in popularity, such as neonatal PDA stenting, whereas older interventions like ballooning of narrowed valves have become well established. The success rate is rising as operators’ experience is increasing. These procedures are safe, effective, and are an attractive alternative to cardiac surgery. In some circumstances they have replaced surgery and in other situations they provide a bridge to surgery or a useful adjunct. Device closures and valve replacement have supplanted surgery in many countries, but the major constraint in some areas remains the cost of the devices and the lack of tertiary care centres that are dedicated to such procedures and have the trained personnel. The goal of reaching the perfect device/procedure still leaves much to be accomplished in the future.








