Abstract
Background: Coronary artery disease (CAD) includes a wide spectrum of entities beyond the atherosclerotic disease. Coronary artery fistulas (CAF) represent an uncommon vascular abnormality that may cause several cardiovascular complications and symptoms, due to the coronary steal phenomena. Surgical or percutaneous closure should always be considered. The authors present a case series of patients with CAFs who developed cardiovascular manifestations, and underwent percutaneous closure safely and feasibly, with good clinical results.
Case Summary: Five patients with CAFs were treated from 2021–2023; three were male (60%), the mean age was 59 years, the most common symptom was chest pain, and two patients presented in the context of unstable angina. The authors documented pulmonary hypertension in three patients, none of them with haemodynamic compromise of right ventricle. Two of the patients had documented ischaemia or haemodynamic significance due to the CAF. Finally, in two cases, no CAD was noted in coronary angiography. Percutaneous closure was done using a 6 Fr or 7 Fr sheath; guiding catheter 6 or 7 Fr through a workhorse guidewire, a microcatheter was placed in the coronary origin of the fistula, closure was done using a liquid embolisation system or delivering coils into the defect. The number and length of coils may vary depending on the fistula’s size.
Discussion: The authors present five successful cases of percutaneous closure of symptomatic CAF, who presented with angina or dyspnoea as main symptoms. Once the diagnosis was made and further studies performed, the closure was decided based on the pulmonary hypertension or coronary steal phenomena.
Key Points
1. Coronary artery fistulas (CAF) are abnormalities that may lead to several cardiovascular complications depending on the size, flow, and anatomy of the defect.2. The closure of the CAF can be performed percutaneously or surgically, with the latter being indicated if there is another condition requiring it.
3. The percutaneous closure of CAF is a safe intervention, and should be considered based on the size and symptoms associated with the defect.
INTRODUCTION
Coronary artery fistulas (CAF) are communications between a coronary artery and a major vessel or a cardiac chamber.1 More than 90% of CAFs have a congenital or sporadic presentation; nevertheless, coronary stent placement, heart surgery, chest irradiation, or coronary vessel diseases may lead to an acquired CAF.2 The prevalence is estimated in over 0.3% of congenital heart diseases.3 CAFs in adults are mostly asymptomatic, but the symptoms may vary depending on the size and haemodynamic compromise of the defect, varying from asymptomatic forms to dyspnoea and angina.4
In adults, closure is recommended in CAFs larger than two times the size of the normal vessel, regardless of symptoms; and medium or small when the patient develops symptoms like pulmonary hypertension, myocardial ischaemia, ventricular dysfunction, infective endocarditis, or cardiac arrythmias.4,5 Procedural devices vary from vascular plugs, duct occluders, detachable balloons, coils, covered stents, and chemicals, and the choice depends on the size of the defect, the clinical experience, and the delivery approach.6
Due to the lack of evidence, decision to treat may be difficult. Also, it is debatable whether the therapeutic approach should be surgical or percutaneous. Today, surgical closure is preferable in patients with other surgical intervention needed (valve replacement, coronary artery bypass grafting, tortuous or large defects).
Percutaneous closure is associated not only with less surgical complications (bleeding, infections, ischaemia, etc.), but also with fewer recurrence rates.5
This report included five patients who reported different cardiovascular symptoms and underwent coronary diagnostic angiogram. CAF was incidentally diagnosed (patient characteristics can be seen in Table 1).
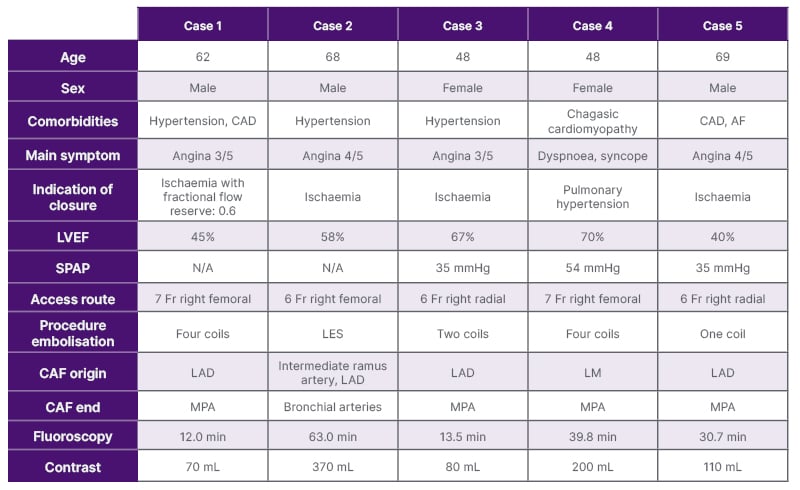
Table 1: Main clinical characteristics of the patients.
AF: atrial fibrillation; CAD: coronary artery disease; CAF: coronary artery fistula; LAD: left anterior descending artery; LES: liquid embolic system; LM: left main trunk; LVEF: left ventricle ejection fraction; min: minutes; MPA: main pulmonary artery; N/A: not applicable; SPAP: systolic pulmonary artery pressure.
Closure decision was made by the cardiology team, depending on the symptoms and haemodynamic compromise of each defect, documented through diagnostic methods from transthoracic echocardiogram, non-invasive coronary stratification, or invasive fractional flow reserve measurement. Coronary anatomy before and after the procedure are illustrated in Figure 1 and Figure 2.
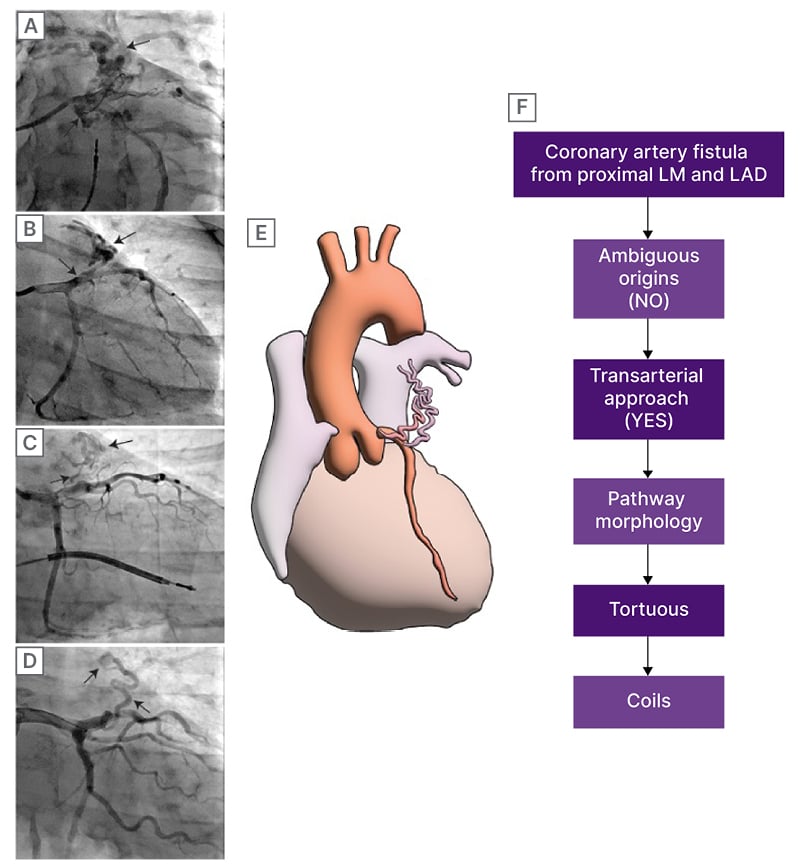
Figure 1: Angiographic characterisation of the CAF and the recommended therapeutic strategy.
A) Coronary angiogram of a CAF whose coronary origins are intermediate ramus and left anterior descending artery, and ends in bronchial artery. B–D) CAF from patients from LAD to MPA. E) Diagram of a CAF from LAD to MPA. F) Flowchart of decision to treat, according to Al-Hijji et al.4
CAF: coronary artery fistula; LAD: left anterior descending; LM: left main.
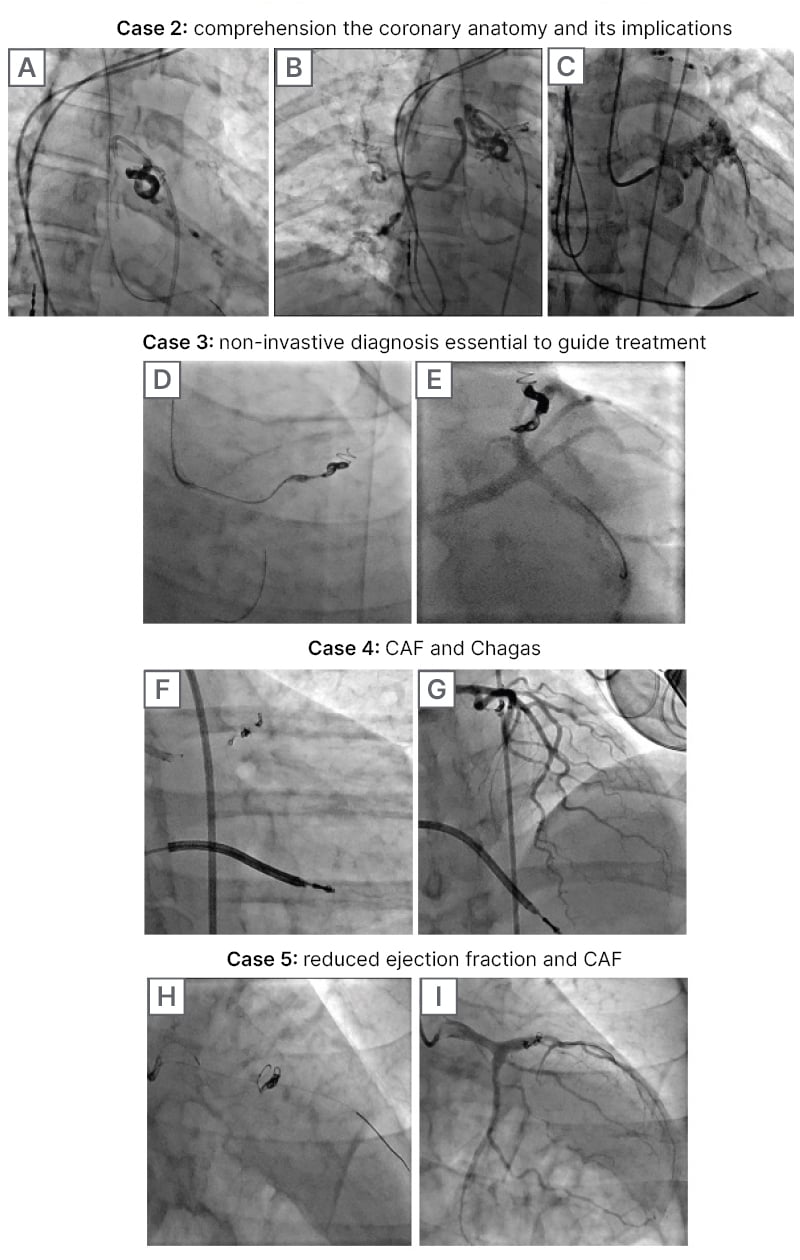
Figure 2: Successful percutaneous embolisation of CAF using coils and liquid embolic system.
A) CAF from intermediate ramus and LAD to bronchial artery closure procedure with LES. B) Preserved flow to bronchial artery after closure of the CAF (TIMI 3 flow). C) Preserved flow to coronary artery after closure of the CAF (TIMI 3 flow). D) CAF embolisation from LAD to MPA using two coils with sizes 4 mm x 15 cm and 7 mm x 30 cm. E) Successful closure of the CAF and preserved flow to the coronary artery (TIMI 3 flow). F) CAF embolisation from LM to MPA using four coils with sizes 1.5 mm x 3.0 cm, 1 mm x 3 cm, 2 mm x 4 cm, and 1 mm x 4 cm. G) Successful closure of the CAF and preserved flow to the coronary artery (TIMI 3 flow). H) CAF embolisation from LAD to MPA using a single 3 mm x 10 cm coil. I) Successful closure of the CAF and preserved flow to the coronary artery (TIMI 3 flow).
CAF: coronary artery fistula; LAD: left anterior descending; LES: liquid embolic system; LM: left main; MPA: main pulmonary artery.
TIMELINE
1. Patients present to the hospital with symptoms and clinical findings that may suggest CAD.
2. Diagnostic coronary angiogram is performed with minimal use of contrast to obtain adequate images of coronary artery anatomy and diagnosing CAF.
3. Decision to treat is made within the cardiology team, based on the compromise of the defect.
4. Percutaneous CAF closure is performed to embolise the fistulas. Techniques and supplies may vary, depending on the size and placement of the defect.
CASE PRESENTATIONS
Case 1: Multimodal Invasive Diagnosis and Coronary Artery Fistulas
A 62-year-old male with a previous psychiatric disorder, arterial hypertension, and CAD presented with chest pain and shortness of breath. Transthoracic echocardiogram documented an ejection fraction (EF) of 45%, with no other significant findings. Coronary angiography documented a CAF from left anterior descending artery (LAD) to main pulmonary artery (MPA) and previously implanted stent in LAD. Intravascular ultrasound showed a mild stenosis in left main trunk with minimal lumen area in 10 mm2; no stent restenosis or disease progression was documented.
Giving the clinical manifestations, and despite the absence of progression of atherosclerotic disease evaluated by intracoronary imaging technique, the authors decided to assess the haemodynamic compromise of the CAF, and thus define the need of intervention at this level. Therefore, they advanced a pressure wire to LAD and performed fractional flow reserve distal and proximal to CAF, revealing haemodynamic significance due (0.69). Procedure was done using a 7 Fr sheath and a 7 Fr guiding catheter; through a workhorse guidewire, a microcatheter was placed in the origin of the CAF, then four coils were delivered to perform the successful closure (coil sizes: 2 mm x 8 cm, 2 mm x 4 cm, 3 mm x 8 cm, and 4 mm x 12 cm).
In further follow-up, the patient referred symptom improvement. No further reintervention was required, and no longer coronary percutaneous interventions have been needed to date yet.
Case 2: Comprehension of the Coronary Anatomy and its Implications
A 68-year-old male with history of sinus node dysfunction, arterial hypertension, and CAD came into the emergency department, presenting with an unstable angina. Transthoracic echocardiogram documented a preserved EF (58%), with no wall motion defects or severe valve diseases. Coronary angiography evidenced two CAFs, whose coronary origin were intermediate ramus and left anterior descending arteries to bronchial artery. It was decided to perform a percutaneous closure of the CAF to avoid coronary steal phenomena. Closure was performed using a 6 Fr sheath, 6 Fr guiding catheter, a workhorse guidewire, and a microcatheter was placed in the bronchial artery’s origin. The authors delivered a liquid embolic system with successful closure of the CAF, remaining distal flow to the bronchial artery. To date, the patient remains asymptomatic, without another reintervention.
Case 3: Non-invasive Diagnosis Essential to Guide Treatment
A 48-year-old female with history of arterial hypertension was referred to the hospital due to chest pain and bradycardia. A myocardial perfusion study revealed severe ischaemia of the anterior wall. Transthoracic echocardiogram evidenced a preserved EF (67%), and systolic pulmonary artery pressure (SPAP) of 35 mmHg without haemodynamic compromise of right heart chambers. The patient underwent coronary angiography with no evidence of CAD, but a CAF from LAD to MPA. Given the results of the non-invasive study, it was determined that the CAF was generating coronary steal to the anterior wall, and it was considered that it should be treated. Percutaneous closure was performed using a 6 Fr sheath, 6 Fr guiding catheter, and a workhorse guidewire; a microcatheter was placed into the coronary origin of the CAF and then, two coils were delivered (4 mm x 15 cm, and 7 mm x 30 cm) to embolise the fistula. At follow-up, the patient remained asymptomatic, with a quality of life improvement, and no further interventions required.
Case 4: Coronary Artery Fistulas and Chagas
A 48-year-old female with history of Chagasic cardiomyopathy with an implantable cardioverter defibrillator presented to the emergency department with shortness of breath and syncope. Echocardiogram showed a preserved EF (70%), SPAP was 54 mmHg, and no valve disease or apical aneurysm was noted. No coronary atherosclerotic artery disease was documented in coronary arteriography, but there was a CAF from left main trunk to MPA. Despite pharmacological management and rehabilitation, the patient persisted symptomatic with New York Heart Association (NYHA) Stage III/IV. It was decided to perform percutaneous closure of the CAF. The authors performed percutaneous CAF embolisation using a 7 Fr sheath, 7 Fr guiding catheter, and a workhorse guidewire. They placed a microcatheter into the coronary origin of the CAF, and delivered four coils there (1.5 mm x 3.0 cm; 1 mm x 3 cm; 2 mm x 4 cm, and 1 mm x 4 cm). After discharge, she remained asymptomatic with NYHA I/IV.
Case 5: Reduced Ejection Fraction and Coronary Artery Fistulas
A 69-year-old male with previous CAD and atrial fibrillation presented to the hospital due to an unstable angina. Echocardiogram documented a moderated reduced EF (40%), SPAP of 35 mmHg, and a severe functional mitral insufficiency. During stress-echocardiography, inferior wall ischaemia was documented. Coronary angiography documented CAD in PDA, and a CAF from LAD to MPA. Given the context of the patient, with compromised systolic function added to the CAF in the LAD, and severe coronary disease in a PDA, the cardiology team defined percutaneous intervention of the CAD and CAF closure. The authors performed the intervention with a 6 Fr sheath, 6 Fr guiding left and right catheter, and, with a workhorse guidewire, placed a drug-eluting stent in PDA. They then advanced the guidewire to LAD, and placed a microcatheter in the CAF, delivering a single 3 mm x 10 cm coil with successful closure. At follow-up, the patient was cardiovascular asymptomatic, without coronary reintervention or hospitalisation.
DISCUSSION
CAFs are rare congenital or acquired abnormalities, associated to a wide spectrum of cardiovascular diseases. CAFs are classified depending on the anatomy of the defect, its origin and termination, its morphology and size, and its physiologic abnormalities. The most common CAF is coronary-pulmonary artery fistula. Other less common terminations are pulmonary veins, venae cavae, coronary sinus, bronchial vessels, or even coronary cameral fistulas.7
CAFs remain asymptomatic most of the time. However, they may lead to several unspecific symptoms, such as pulmonary hypertension, endarteritis, or myocardial ischaemia, whether due to early atherosclerotic CAD or coronary steal phenomena in the absence of CAD.1 As seen in previous cases, the haemodynamic compromise generated by the CAF has been corroborated with both invasive and non-invasive studies.
As the majority of CAFs remain asymptomatic, closure decision is controverted. Current evidence suggests closure should be considered when the CAF becomes symptomatic (documented ischaemia, ventricular dysfunction, cardiac arrythmia, or endarteritis), and its size is at least 1–2 times bigger than the normal size of the origin vessel; or when vessel aneurysm or rupture risk is present.4 Coronary-pulmonary artery fistula is indicated when a left-right shunt is documented.8 In the authors’ cases, the patients reported cardiovascular symptoms, and haemodynamic compromise was documented by invasive and non-invasive studies, with LVEF reduction and pulmonary hypertension, with clear clinical improvement without reintervention after the therapeutic intervention.
Transcatheter closure is the most suitable choice in most of the cases, due to its minimal invasive techniques. Surgical closure should be only reserved for those patients who are meant to go into a concomitant cardiac surgical intervention, and estimate mortality risk is lower than 10%.7 Several percutaneous closure techniques have been described, depending on the access choice. The required equipment list includes 0.014 and 0.035 hydrophilic intracoronary wires, multipurpose catheters, microcatheters, and more.3 The authors’ percutaneous strategy, according to the anatomical characteristics, was performed by femoral access with 6 to 7 Fr according to the desired support. The usual workhorse guidewire and microcatheter were used to release the device or closure liquid. The authors’ experience, as well as experience in previously published literature, demonstrate that the use of a microcatheter through a 6 Fr or 7 Fr sheath, and the use of a coronary guidewire, may be sufficient to perform a successful percutaneous coil closure.9,10
As a case series manuscript, there are some limitations to declare. The first is due to the low incidence of the disease, which means that no large, randomised studies with statistic power are feasible. Also, confusion bias may be caused due to previous or concomitant cardiac diseases, such as CAD, sinus node dysfunction, atrial fibrillation, or Chagas disease.







