INTRODUCTION
Obesity is a pandemic. The prevalence of obesity (BMI >30) and morbid obesity (BMI >40) among American adults is approximately 30% and 5%, respectively.1 The condition is strongly associated with medical comorbidities (e.g., heart disease, Type 2 diabetes mellitus, stroke, obstructive sleep apnoea, certain types of cancer, and osteoarthritis) and mortality, and has begun to overtake infectious diseases as the most significant contributor to illness worldwide. Furthermore, it is a leading preventable cause of death worldwide, with increasing prevalence in adults and children, and considered one of the most serious public health problems of the 21st century.2
Currently, there are three clinically viable treatment options for obesity: surgery, pharmacologic intervention, and intragastric balloon administration. However, these methods have varying success rates and are not free of complications. There remains a critical need for a minimally invasive intervention that can target this growing population.
GASTROINTESTINAL HORMONES AND OBESITY
Obesity often presents as a combination of several component causes, including excessive caloric intake, a sedentary lifestyle, genetic susceptibility, or hormonal imbalances. There has been a growing understanding of the critical involvement of the stomach as an endocrine organ in the maintenance of energy homeostasis. Among more than 40 hormones that have been discovered to limit food intake, one hormone, ghrelin, is orexigenic (i.e., stimulates food intake). Ghrelin plays a role in both short-term and long-term food regulation. Cells producing ghrelin are predominantly located in the fundus of the stomach and directly fuel appetite and induce a positive energy balance, resulting in body weight gain.3
INTERVENTIONS TO MODULATE GHRELIN LEVELS
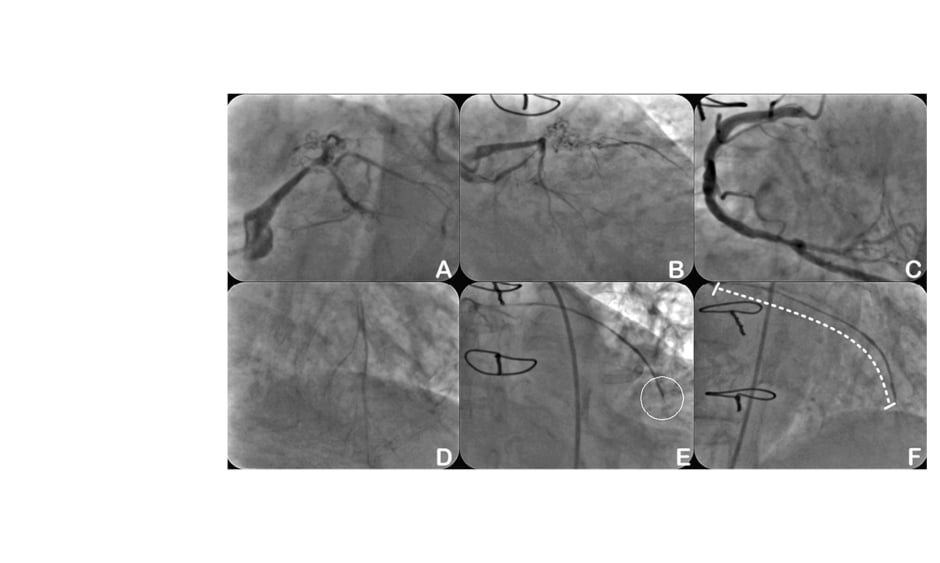
Varying attempts, whether mechanical or pharmaceutical, to modulate ghrelin production with the aim of food intake reduction have been undertaken, but significant clinical results have yet to be achieved. However, ghrelin is a promising target in both medical and surgical approaches to obesity. Recently, a number of animal studies have demonstrated that embolising the arteries supplying the gastric fundus, a major source of ghrelin, can lead to a reduction in blood ghrelin levels and subsequently promote reduced food intake.4-6 In 2012, the authors performed the first-in-human embolisation of the left gastric artery or transcatheter bariatric embolisation (TBE) in five obese patients using off-the-shelf equipment and 300–500 μm compressible microspheres (Biocompatibles UK Limited, Surrey, UK).7 Based on the authors’ previous animal studies (unpublished data), using particles with sizes ranging 300–500 μm is advantageous because smaller particles (e.g., 50–100 μm) can result in mucosal necrosis of the fundus and produce gastric ulcers. The first-in-human study demonstrated a mean weight reduction of 16% at 6-months follow-up. Blood ghrelin levels were significantly lower at 1- and 6-month follow-up (by 29% and 36% from baseline; p<0.05) and increased at 6-month follow-up compared with 3-month follow-up, while remaining 18% lower than the baseline (p<0.05).7
Following the first-in-human study, there have been several human clinical studies evaluating the effects of gastric embolisation for weight loss.8,9 These studies have demonstrated clinically meaningful weight-loss results post-embolisation. They were, however, non-randomised, single-arm feasibility, safety, and efficacy trials, which come with inherent limitations. First, the absence of a control group does not allow definitive conclusions regarding efficacy. It is possible that the procedure and study participation led to a higher motivation for diet control and exercise. However, a decrease in plasma ghrelin levels should not be expected. Second, the intermediate-term follow-up is too short to allow conclusions regarding long-term weight loss. A rebound phenomenon with recurrent weight gain is conceivable. Third, in most of these studies there is a report of significant gastric ulcer formation, not attributed to the fundus but instead non-target embolisation. Using special antireflux microcatheters may limit antegrade and retrograde reflux and therefore minimise non-target embolisation: the current Achilles’ heel of embolisation procedures performed in these highly collateralised tissues.
CARDIOVASCULAR COMORBIDITIES IN OBESE PATIENTS
In the landscape of approved obesity treatments, most, if not all, interventional approaches (surgical or pharmaceutical) require the presence of a comorbid condition. Numerous studies have indicated the correlation between obesity and heart disease or Type 2 diabetes mellitus. Obesity has also been linked to heart failure and atrial fibrillation (AF) and poorer prognosis in obese patients, with research demonstrating an association between increased visceral and epicardial fat depots. Recurrence rates following AF ablation are also higher in obese patients. A 5–10% weight loss has been associated with clinically meaningful reductions in HbA1c, triglycerides, low-density lipoprotein cholesterol, and as much as a 5–10 mmHg reduction in systolic blood pressure. The weight-loss targets are well within the weight loss achievable through TBE.
CONCLUSIONS
A randomised controlled trial with mid-term follow-up has recently demonstrated clinically significant weight loss in TBE over a sham group.10 Further studies with larger patient numbers and long-term follow-up are needed to examine the utility of this procedure. Widened experience with this procedure will provide a refinement in procedural technique and use of dedicated antireflux devices to maximise the effect of weight loss but minimise the risk of mucosal injury and ulcer formation.
TBE may be utilised in the general population, however, a large proportion of patients who are in the catherisation lab for a percutaneous coronary, peripheral, structural, endovascular intervention, or AF ablation are also obese or overweight. These patients rarely visit specialised bariatric or weight-loss clinics. Therefore, percutaneous TBE could be a possible technique to add to the tool kit of the interventional cardiologist and radiologist who are already treating this patient population. As in structural heart disease, the authors recommend the creation of a weight-loss team consisting of a general cardiologist, interventional specialists, bariatric surgeon, registered dietitian, and endocrinologist or weight-loss specialist.