Abstract
Percutaneous coronary intervention (PCI) is the most common form of revascularisation in patients with coronary artery disease in both the elective and acute coronary syndrome settings. Advances in pharmacotherapy have reduced ischaemic complications and improved outcomes in PCI, albeit at the expense of major bleeding. Major bleeding complications are amongst the most common to occur following PCI, with varying incident rates reported due to different definitions of what constitutes a ‘major bleeding event following PCI’, and the risk profile of the patients studied. Irrespective of the bleeding definition used, major bleeding events universally lead to a worse outcome. Major bleeds can occur at both the access site used for PCI and non-access site sources. Both access site and non-access site bleeding increase mortality following PCI. Patients who undergo PCI are at an increased risk of bleeding for several years following the procedure. Strategies to reduce the risk of bleeding should focus on pharmacotherapy, and importantly, use a radial rather than femoral approach to perform PCI.
INTRODUCTION
Cardiovascular disease causes >4 million deaths in Europe annually, and coronary artery disease is responsible for the majority of these.1 Treatment of coronary artery disease with percutaneous coronary intervention (PCI) is increasing due to the presence of an ageing population and better access to specialist healthcare services.2 PCI has become increasingly safe, with in-hospital mortality falling from up to 5% in the 1980s3 to <1% in contemporary practice,4 however bleeding following PCI remains a major cause of both morbidity and mortality.5 This review article provides an overview of bleeding in the setting of PCI, exploring the definitions, frequency, sites, timing, and prognostic impact of bleeding in PCI patients, as well as strategies to minimise the risks of bleeding.
DEFINITION OF MAJOR BLEEDING
Reports of major bleeding in patients undergoing PCI procedures is variable, ranging from 1–10% in the literature.6 This wide variability in reported incidence of bleeding is due to a variety of factors, including differences in patient populations, antithrombotic therapies, the nature of the intervention performed, and importantly, the definition of bleeding that is used. There is currently no universal definition of major bleeding, and there are >10 different definitions used in clinical trials and registries.7 These definitions rely on different combinations of laboratory measures (a decrease in haemoglobin [Hb] or haematocrit) and clinical events (need for transfusion, haematoma, tamponade, need for surgery, etc.).
One of the most widely used definitions over the last three decades has been the Thrombolysis in Myocardial Infarction (TIMI) definition of bleeding.8 This definition was initially developed to categorise bleeding into major and minor events within the context of patients undergoing thrombolytic therapy to treat ST-elevation myocardial infarction (STEMI), and relied largely on tested laboratory values of Hb and haematocrit. Criticism of the definition centred on both the values selected for major bleeding (a decrease in Hb of >5 g/dL), and the lack of focus on clinical events. The original definition has been refined over time to reflect these observations with a broader range of bleeding categories. The Global Use of Strategies to Open Occluded Arteries (GUSTO) definition of bleeding has also been widely used.9 Again this definition was originally developed in the context of thrombolysis in STEMI patients, but its grading of the severity of bleeding relied on an assessment of the clinical impact of the bleed. Importantly, it did not require changes in laboratory Hb levels, and did not quantify the size of any required blood transfusion. A comparison of outcomes in acute coronary syndrome (ACS) patients, using these distinct definitions, suggests that whilst both are good at predicting adverse outcomes in the acute setting, the risk of GUSTO-defined bleeding persists whilst that of TIMI does not.10 Attempts have been made to combine the benefits of the TIMI and GUSTO definitions of bleeding to overcome their deficiencies.11
The Bleeding Academic Research Consortium (BARC) have also made efforts to create a universal bleeding definition.7 The initiative developed a definition that captures the nature of bleeding complications, correlating this with prognosis to help guide diagnostic and treatment protocols. The BARC definition grades events from no bleeding (Type 0) up to fatal bleeding (Type 5). Early evidence suggests that this definition is a useful addition to clinical trials, demonstrating good correlation with clinical outcome. The definition of bleeding is vitally important as it can determine the outcome of a study. The landmark RIVAL study did not find a significant difference in major bleeding between the radial and femoral access sites.12 This study however used its own definition of major bleeding (the need for transfusion of >2 units of blood, hypotension requiring inotropes, a drop in Hb of >50 g/dL, intracranial bleeding), which has limitations. Using a broader definition of bleeding (such as that used in the ACUITY trial)13 would have resulted in radial access being associated with a significant reduction in major bleeding. Similarly, the MATRIX study used a BARC definition of bleeding and reported a significant reduction in major bleeding episodes associated with radial artery access.14 Their analysis also reports that use of TIMI or GUSTO major bleeding definitions would have resulted in no significant difference in rates of major bleeding between the two groups. Table 1 summarises the differences in bleeding definition between TIMI, GUSTO, and BARC.
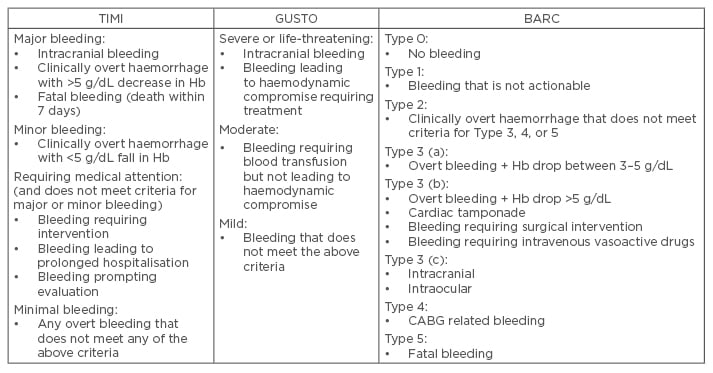
Table 1: Different definitions of bleeding.
TIMI: thrombolysis in myocardial infarction; GUSTO: Global Use of Strategies to Open Occluded Arteries; BARC: Bleeding Academic Research Consortium; CABG: coronary artery bypass graft; Hb: haemoglobin.
WHY IS BLEEDING IMPORTANT?
Regardless of the definition used, bleeding is associated with a significant increase in the risk of death, myocardial infarction (MI), and cerebrovascular accident (CVA).5,15 Up to 12% of deaths following PCI may relate directly to bleeding complications.16 Recently, strong evidence has emerged that bleeding is independently associated with a higher risk of death. A large meta-analysis by Kwok et al.17 including 42 studies and >500,000 patients found that bleeding was an independent predictor of death. They found that in studies where adjustments were not made for confounding comorbidities, bleeding was associated with a 6-fold increase in risk of death. Studies in which these comorbidities had been factored in were still associated with a 3-fold increase in risk of death. Importantly, the definition of bleeding also had an impact, with a hazard ratio (HR) for death in the range of 1.5–6.7 depending on the definition used.
There are different mechanisms by which major peri-procedural bleeding has an outcome on long- term survival. A major bleed can lead to morbidity and mortality due to location (for example intracranial) or volume of blood loss leading to hypovolaemic shock. Different types of bleeding can have varying impact on outcomes. A pooled analysis by Ndrepepa et al.18 of 12,459 patients undergoing PCI in six studies, found a close association between BARC-defined bleeding Class ≥2 and 1-year mortality. BARC Class ≥3 was even more strongly associated with 1-year mortality (HR: 3.2, confidence interval [CI]: 2.3–4.4, p<0.001) than BARC Class ≥2 (HR: 2.7, CI: 2.0–3.6). Another prospective cohort study by Matic et al.19 also found a relationship between different BARC classes of bleeding and mortality. Of the 1,808 patients with a STEMI, 1-year mortality increased from 11.5% in BARC Class 0 and 1 to 43.5% in BARC Class 3b bleeding. Both BARC 3a bleeding (HR: 2.0, CI: 1.2–3.4, p=0.012) and BARC 3b bleeding (HR: 3.2, CI: 1.7–6.2, p<0.0001) were independently associated with death at 1 year. Some of the trials examining major bleeding and mortality are listed in Table 2.
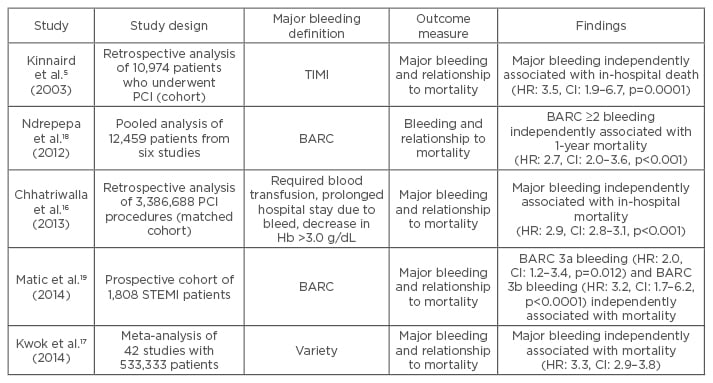
Table 2: Selected studies examining major bleeding and mortality outcome.
Hb: haemoglobin; HR: hazard ratio; CI: confidence interval; BARC: Bleeding Academic Research Consortium; PCI: percutaneous coronary intervention; STEMI: ST-elevated myocardial infarction; TIMI: thrombolysis in myocardial infarction.
Bleeding and anaemia can also lead to an increase in the production of erythropoietin, a substance that is known to have prothrombotic and platelet-activating effects.20 Treatment with erythropoietin in patients following MI has been shown to be associated with an increased risk of further MI, CVA, and death.21 This hypercoaguable state is a particular risk for patients who have had PCI and may have coronary stents. Bleeding (and the perceived increased risk of recurrent bleeding) may lead to alteration in antiplatelet regimes. Discontinuation of antiplatelets is strongly associated with stent thrombosis, MI, and death.22 Bleeding may also lead to discontinuation of warfarin in patients with concurrent atrial fibrillation (AF) and this has been shown to be associated with significantly higher rates of death.23
PREDICTORS OF BLEEDING
Unsurprisingly, the risk of bleeding increases as patients become older, present with an ACS, or have renal or heart failure.24 The risk of bleeding at 30 days following PCI increases from between 0.7% and 1% in elective patients25 to 4.7% in non-STEMI patients26 and 8.9% in STEMI patients.6,27 The predictors of early bleeding are often procedure-related such as access site, sheath size, and antithrombotic regimen, in addition to comorbidities.28 These are less likely to have an influence on late bleeding. Independent predictors of late bleeding include age, previous bleeding episode, chronic kidney disease, and triple therapy with dual antiplatelets and warfarin.29
WHERE DO PATIENTS BLEED?
Major bleeding following PCI can occur at different locations. Bleeding can occur at the radial or femoral artery access site (and spread to adjacent tissue), or at non-access sites such as the gastrointestinal (GI) tract, the pericardium, the pulmonary system, etc. A large meta-analysis by Kwok et al.17 showed that non-access site bleeding is common and is responsible for between half and two-thirds of all TIMI classification bleeding events. Across seven studies, with a combined total of 301,404 patients, access site-related bleeding complications had a prevalence of 11.2%. Across six studies with 290,456 patients, non-access site bleeding had a prevalence of 10.2%. Although both access site and non-access site bleeding are associated with increased mortality, non-access site bleeding is more closely correlated with adverse outcomes, and is associated with a 4-fold increase in 1-year mortality.30 The meta-analysis by Kwok et al.17 corroborated these findings and showed that whilst access site bleeding significantly increased the risk of 30-day mortality (relative risk: 1.71, 95% CI: 1.37–2.13), the increase in risk following non-access site bleeding was much greater (relative risk: 4.06, 95% CI: 3.21–5.14). In patients who have non-access site bleeds, the anatomical location can also have an impact on outcome. The same meta-analysis of studies involving non-access site bleeding found a mortality of 13% in patients after a bleed into the GI tract, 6.8% after a retroperitoneal bleed, 56% following an intracranial bleed, and 8.6% following an intramyocardial or pericardial bleed. Haemorrhagic CVA is of particular concern as it is associated with very significant 30-day mortality (odds ratio: 13.87, 95% CI: 6.37–30.21).31 A large meta-analysis found that the most frequent location of non-access site is the GI tract (20.4%), followed by genito-urinary tract (7.7%), with intra-cranial bleeding occurring in only 0.5% of the population.32
WHEN DO PATIENTS BLEED?
Patients undergoing PCI are at risk of peri-procedural bleeding but continue to be at excess risk for some time following this. In the CREDO trial, which followed patients who had undergone PCI, patients were randomised to receive clopidogrel and aspirin for either 1 month or 1 year, and the incidence of non-procedural major bleeding in the prolonged clopidogrel group was 1.2%.33 In the PCI subgroup of the CURE study, major bleeding occurred in 1.1% of patients between 1 and 9 months in those randomised to aspirin and clopidogrel.34 Real-world data suggests that in patients >65 years, major bleeding rates may be as high as 2.5% in the year following PCI in those treated with dual antiplatelets.29 To place these rates of bleeding into context, patients with AF on warfarin have an incidence of major bleeding of 1.3% per year and 2.2% per year on warfarin plus aspirin.35 Recent work from the ADAPT-DES trial reported on bleeding events related to PCI after hospital discharge and up to 2 years.36 They found that the risk of bleeding accrues constantly with rates of first bleeding at 30 days, 1 year, and 2 years of 0.7%, 3.8%, and 8.8%, respectively. This is an important message as it reiterates that patients who undergo PCI continue to be at significant risk following discharge from hospital. The same study found that a majority of bleeding events after hospital discharge are related to the GI tract (61.7%), and that following multivariate analysis, post-discharge bleeding was the strongest predictor of 2-year mortality.
The increased risk of death associated with bleeding may not be limited to the immediate hospital admission. Although bleeding following PCI is strongly associated with in-hospital and 30-day mortality, the evidence of an impact on longer-term outcomes is less clear. The TRITON-TIMI 38 analysis found that although major bleeding was strongly associated with mortality within the first month of PCI, the association was not significant beyond 40 days.37 However, other groups have found that major bleeding is associated with increased mortality at 6 months,38 1 year,29 and even 3 years following PCI.39
BLOOD TRANSFUSION
Anaemia is independently associated with an increased risk of MI and cardiac mortality in patients undergoing PCI.40 The use of blood transfusion to correct anaemia and to treat bleeding remains controversial. Around 2% of all patients treated with PCI undergo a blood transfusion, either following an acute bleeding event or due to chronic anaemia.41 In patients who present with an ACS who undergo PCI, the rates of blood transfusion may be as high as 10%.42 Although there is great variation in practice regarding the use of blood transfusion, women, the elderly, patients with renal or heart failure, and those with a history of prior ischaemic heart disease are most likely to receive a blood transfusion.43
Regardless of whether a patient has a bleeding episode following PCI, the need for a blood transfusion is associated with up to a 3-fold increase in rates of MI or death in patients who present with an ACS.43 A previous meta-analysis of >200,000 patients presenting with myocardial infarction that compared a liberal blood transfusion policy (defined as transfusion for Hb <9.0 g/dL) versus a more restrictive policy (transfusion for Hb <7.0 g/dL) suggested that a liberal blood transfusion policy was associated with increased all-cause mortality.44 This study involved a majority of patients who were managed medically rather than following PCI so it is less relevant to contemporary practice. A more recent meta-analysis involving >2 million patients who had undergone PCI, examined the impact of blood transfusions on outcome.45 It confirmed that blood transfusion following contemporary PCI is relatively common, with a prevalence of 2.3% (>54,000 patients received a transfusion). The study also found that blood transfusions were an independent predictor of major adverse cardiac events and death, increasing the risk by as much as 3-fold. Importantly, blood transfusions are associated with a worse outcome even in patients who do not have a bleeding event.5 The absence of clear evidence from randomised controlled trials in the area of PCI and blood transfusion have led to a lack of clear guidelines as to when blood transfusion should be used in the setting of PCI and ACSs.46
BLEEDING RISK
As the importance of bleeding to outcome following PCI has become clear, models have been developed to help quantify the risk of bleeding. Mehta et al.24 performed an initial analysis of 302,152 patients from the National Cardiovascular Data Registry (NCDR), and proposed an algorithm featuring age, sex, previous heart failure, renal impairment, peripheral vascular disease, previous PCI, heart failure class, presence of ST elevation, and cardiogenic shock to categorise patients into low, intermediate, or high-risk of bleeding. This was further improved after an analysis of 1,043,759 PCI procedures from the NCDR CathPCI registry by Rao et al.47 The new NCDR model used 10 clinical variables to give a point score between 0 and 210 that predicted a bleeding risk of between 0.9% and 86% following PCI. An alternative model is the CRUSADE bleeding score developed by Subherwal et al.48 and based on 71,277 non-STEMI patients who underwent PCI. This model uses clinical variables to give a score of between 1 and 100 points to grade risk of bleeding. This and other risk scoring systems are limited by the requirement for laboratory values, which may not always be available prior to emergency situations such as a primary PCI. Doubts have also been raised about the predictive value of scoring systems for bleeding in patients undergoing PCI.49
STRATEGIES TO REDUCE RISKS OF BLEEDING
Major bleeding complications are associated with significant immediate and long-term morbidity and mortality. Every effort should be made to minimise the risk of bleeding in patients undergoing PCI. The primary mechanisms available to achieve this are the adjustment of adjunctive pharmacotherapy, and a pragmatic choice of access site for PCI.
Pharmacology
Antiplatelet agents
The drive to reduce ischaemic complications during and following PCI has led to the development of increasingly potent antiplatelet agents. The benefit of clopidogrel (a thienopyridine) in addition to aspirin in patients undergoing PCI following an ACS was clearly established by the PCI-CURE study,34 in which clopidogrel significantly reduced a composite endpoint of cardiovascular death, MI, and target-vessel revascularisation (HR: 0.70, CI: 0.50–0.97, p=0.03) with no significant increase in major bleeding. The loading dose of clopidogrel can also have an effect on outcome. It has been shown that a 600 mg loading dose of clopidogrel compared with 300 mg reduces mortality, reinfarction, and stent thrombosis with no increase in major bleeding in STEMI patients undergoing PCI.50 The increased loading dose also reduced cardiovascular events without any increase in major bleeding in a group of ACS patients being treated with PCI.51
When compared with clopidogrel, the more potent agent prasugrel has been shown to significantly reduce cardiovascular death, MI, and CVA (HR: 0.81, CI: 0.73–0.90, p<0.001) in 13,608 patients with ACS being treated with PCI,52 however these reductions came with a significant increase in major, life-threatening, and fatal bleeding. Net clinical benefit favoured prasugrel in most groups, but patients with a previous CVA had net clinical harm from prasugrel, and patients aged >75 years and those with a weight of <60 kg obtained no net clinical benefit from prasugrel. Patients who did not have any of these risk factors did not have any additional bleeding with prasugrel compared with clopidogrel. Similarly, treatment with ticagrelor rather than clopidogrel in patients with ACS where an invasive strategy is planned was associated with a significantly lower rate of a composite of cardiovascular death, MI, and CVA (HR: 0.84, CI: 0.75–0.94, p=0.0025).53 Within the GUSTO criteria, ticagrelor was not associated with any increase in severe bleeding compared with clopidogrel.
Oral anticoagulant therapy
If PCI is planned for a patient concurrently taking an oral anticoagulant for another indication (usually AF, mechanical heart valves, or history of thromboembolic disease), antiplatelet therapy with aspirin and a thienopyridine is indicated, but such triple therapies are associated with a significantly higher risk of non-fatal and fatal bleeding.54 The WOEST study randomised 573 patients receiving vitamin k antagonists (VKA) and undergoing PCI to clopidogrel alone or clopidogrel and aspirin (triple therapy) for 1 year.55 The clopidogrel alone group had significantly fewer bleeding events (HR: 0.36, CI: 0.26–0.50, p<0.0001) with no increase in thrombotic events (although the trial was not sufficiently powered to detect this). It should also be noted that femoral access was used in >70% of the patients in the trial. These findings were corroborated by a retrospective analysis where the authors investigated the risk for thrombotic events and bleeding according to multiple antithrombotic regimens after MI or PCI in AF patients.56 At 1 year, there was no increased risk of recurrent coronary events for dual therapy (HR: 0.69, 95% CI: 0.48–1.00) relative to triple therapy, and bleeding risk was also not significantly lower for VKA plus clopidogrel (HR: 0.78, 95% CI: 0.55–1.12) versus triple therapy. The optimum length of triple treatment remains unclear. The risk of stent thrombosis is highest early after PCI and declines with time, whilst the risk of bleeding increases with length of treatment with triple therapy. The ISAR-TRIPLE trial investigated whether 6 weeks of clopidogrel therapy was superior to 6 months of clopidogrel therapy in patients receiving VKA and aspirin following PCI.57 The study found that the primary endpoint (composite of death, MI, CVA, stent thrombosis, major bleeding) was not significantly different between the two groups.
Glycoprotein IIb/IIIa inhibitors
Glycoprotein IIb/IIIa inhibitors (GPI) also have very strong antiplatelet effects with a subsequent reduction in ischaemic events at the cost of increased bleeding following PCI.58 Comparison of heparin and GPI versus bivalirudin showed a net clinical benefit in the bivalirudin group, driven largely by bleeding events in the GPI group.27 More recent work showed no benefit of bivalirudin versus heparin alone.59 The use of low molecular weight heparins in the treatment of ACS is well established. There is evidence that the use of fondaparinux over enoxaparin leads to a similar reduction in ischaemic events with a reduction in major bleeding and subsequently mortality.
When considering treatment, there should be a balance between reducing ischaemic events such as stent thrombosis and myocardial infarction, and bleeding events following PCI. The selection of particular antiplatelet agents and adjunctive pharmacotherapy can be aided by using one of the described bleeding risk scoring systems to assess individual patient risk.
Access Site
Transfemoral PCI has historically been the default access site for many institutions across the world, although transradial PCI had been described as early as 1993. In recent years, the number of procedures carried out via the radial route has increased dramatically, particularly in Europe. Whilst pharmacotherapy has an impact on the rates of bleeding, there is a growing body of evidence that suggests that using a transradial rather than transfemoral approach has a much larger impact in relation to reducing bleeding events and subsequent adverse outcomes.
Analysis of UK data suggests that radial versus femoral PCI in STEMI patients causes significantly fewer access site-related bleeds, and is independently associated with a 30% reduction in 30-day mortality.60 A meta-analysis comprising nearly 3,000 patients similarly found that radial PCI is associated with a significant reduction in mortality compared with femoral PCI, with the result being driven by a reduction in major bleeding events.61 Other work has shown that the biggest reduction in mortality in patients treated using radial PCI occurs in those who are at highest risk of bleeding.62 A recently published large, randomised, multicentre trial comparing radial versus femoral access in ACS patients undergoing PCI also found a reduction in net clinical events including bleeding and mortality.14 Similarly, the RIFLE-STEACS study observed a significant reduction in bleeding and cardiac mortality with radial compared with femoral access.63 By contrast the STEMI-RADIAL trial showed a significant reduction in bleeding and access site complications with radial access but no mortality benefit.64 Although the RIVAL study found no significant benefit in non-STEMI patients, the STEMI subgroup had a significant reduction in bleeding and mortality in the radial access group.65 The findings of these important trials comparing radial versus femoral access are summarised in Table 3. The weight of evidence favouring radial over femoral PCI has led to the European Society of Cardiology (ESC) giving a 1A recommendation to radial over femoral access in patients presenting with an ACS.46
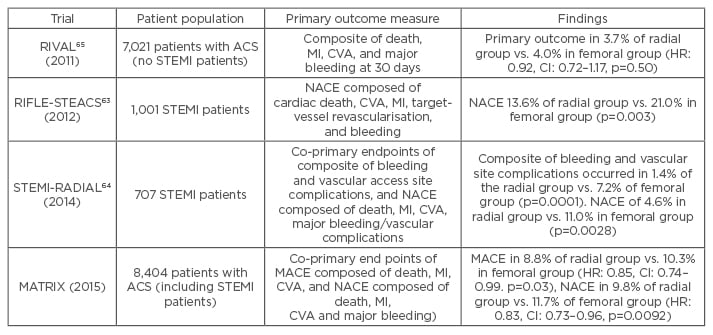
Table 3: Trials comparing radial and femoral access.
RIVAL: Radial vs femoral access for coronary intervention; RIFLE-STEACS: the Radial Versus Femoral Randomized Investigation in ST-Segment Elevation Acute Coronary Syndrome; STEMI-RADIAL: ST-segment elevation myocardial infarction treated by radial or femoral approach in a multicentre randomized clinical trial; MATRIX: Minimizing Adverse Hemorrhagic Events by Transradial Access Site and Systemic Implementation of AngioX Program; MACE: major adverse cardiovascular events; MI: myocardial infarction; NACE: net adverse clinical events; ACS: acute coronary syndrome; HR: hazard ratio; CI: confidence interval; STEMI: ST-elevation myocardial infarction; CVA: cerebrovascular accident.
CONCLUSION
Bleeding following PCI is common and is associated with significant morbidity and mortality. Bleeding should be considered before, during, and after PCI procedures, with a focus on strategies to reduce risk for patients. Pharmacotherapy should be individually tailored to a patient’s risk of ischaemic and bleeding events, and the use of bleeding risk scoring systems can be considered. Radial rather than femoral artery access should be utilised when possible. Blood transfusions should be used judiciously in patients who undergo PCI.








