Abstract
Fetal pericardiocentesis is a safe and effective procedure that is used to drain pericardial effusion in selected fetuses. The aim of the procedure is to reduce the risk of pulmonary hypoplasia, the development of cardiac tamponade and fetal hydrops, and in some cases to allow fetal lung maturity, improving fetal extraction with a better haemodynamic and respiratory condition. In this review, we discuss the indications, technical procedure, and the outcomes of the fetal pericardiocentesis reported in the literature.
INTRODUCTION
The pericardium is a double-walled fibroelastic sac containing the heart and origin of the great vessels. It consists of two layers: an outer or parietal layer and an inner or visceral layer. The pericardium prevents sudden dilation of the heart, especially the right side, and heart and great vessel displacement; it minimises heart friction with the surrounding structures and prevents the spread of infection or cancer cells from the lung and pleura. In between the two layers there is a small amount of fluid, a plasma ultrafiltrate produced by the visceral layer. This liquid facilitates friction-free movement of the heart during systole and diastole within the pericardial sac. During ventricular systole, the pericardial space around the ventricles expands and the pericardial fluid moves into this expanding space, and during ventricular diastole, the pericardial space around the ventricles disappears as the pericardial fluid moves away from the field of view. This phasic movement is characteristically demonstrated by a colour Doppler signal within the pericardial space opposite to the direction of the colour Doppler signal seen within the ventricles.1
PERICARDIAL EFFUSION
Definition
The sonographic finding of fluid with an amount of <2 mm is frequent during ultrasound examination and is seen in >40–50% of normal fetuses, especially when the ultrasound beams are perpendicular to the ventricular walls. This finding has no clinical significance and is not considered to be pericardial effusion.1-4
Pericardial effusion is defined on ultrasound examination when the heart is partially or completely surrounded by liquid that is seen in all projections, is usually present around the atrioventricular groove, and the larger region is >2 mm5,6 and surpasses the atrioventricular union. If it measures <4 mm it is considered small and if it measures >4 mm it is considered large.7 In cases of massive pericardial effusion, a posterior displacement of the lungs can be found on the four-chamber view (Figure 1). The main differential diagnosis is pleural effusion, which appears on sonography, a fluid layer surrounding the lungs.
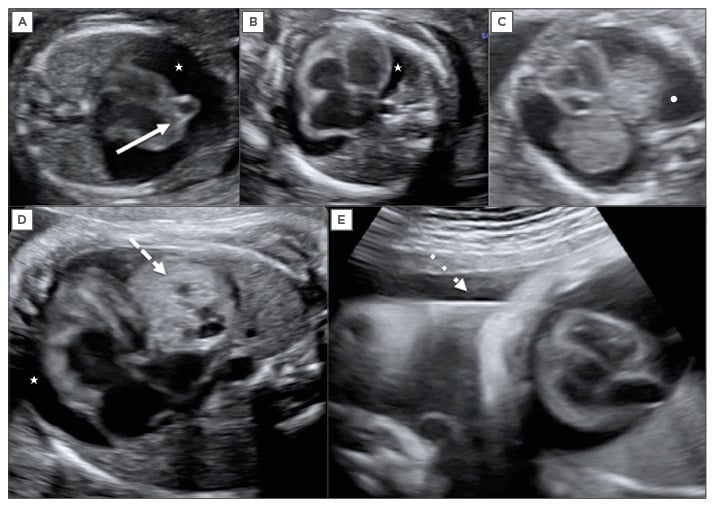
Figure 1: Fetal echocardiography.
A) Large pericardial effusion (white star) associated with fetal cardiac diverticulum at 15 weeks of
gestation (continuous arrow); B) Large pericardial effusion (white star) associated with fetal anaemia; C) Differential diagnosis with pleural effusion showing the fluid around the lungs (white dot); D) Large pericardial effusion (white star) associated with cardiac teratoma at 32 weeks of gestation (discontinuous arrow); E) Pericardiocentesis in this fetus at 32 weeks, the needle crosses the fetal thorax (dotted line arrow).
Aetiopathogenesis
Pericardial effusion can be found isolated or associated with different abnormalities described in the literature (Table 1). The incidence is about 0.64–2.00%.6,8 It is necessary to perform a comprehensive fetal study ultrasound to rule out the different causes to which it is associated. In most cases the effusion occurs as a manifestation of fetal hydrops (immune or non-immune), which is necessary to discard.5 The mechanisms for development of pericardial effusion can be transudative (obstruction of lymphatic drainage, increased lymphatic pressure) or exudative (secondary to inflammation, infection, malignancy, or autoimmune). The pathogenesis depends on the cause.
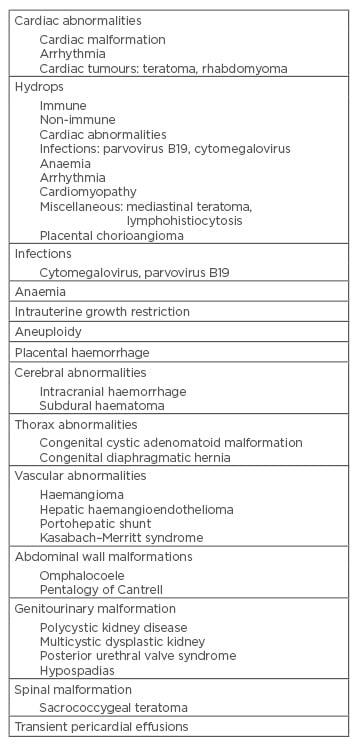
Table 1: Causes of pericardial effusion.
Adapted from Kyeong et al.8
A complete sonographic evaluation of the fetus and maternal blood tests must be performed to determine the presence of abnormal group antibodies, rule out viral infections, fetomaternal haemorrhage, and in some cases amniocentesis for karyotyping and umbilical blood sampling to confirm the presence of anaemia and viral infections. If no causes are found the pericardial effusion is transient and idiopathic, with a better prognosis, and almost 45% of the cases will resolve spontaneously.8
For cases of high-output cardiac failure secondary to fetal anaemia, arrhythmias, or congenital heart disease, studies should be performed to make the differential diagnosis. In cases of fetal anaemia, pericardial effusion is an early sign of hydrops, or appears in a hydropic fetus, which should be treated to resolve the fetal condition.
In the case of cardiac teratomas, the pericardial effusion is probably due to the irritative stimulus of the tumour on the pericardial layers and rupture of the cystic areas in the pericardium caused by the multicystic nature of pericardial teratomas. Furthermore, the tumour can cause mechanical obstruction of the venous return and the thoracic duct which interferes with the lymphatic drainage, leading to the development of pericardial, pleural effusions, ascites, and fetal hydrops. Oesophageal compression can cause polyhydramnios. Pericardial effusion and mass effect are responsible for fetal pericardial tamponade.9
Pericardial effusion is rarely associated with another cardiac tumour. Some cases of cardiac rhabdomyomas with pericardial effusion have been described in literature due to the rapidly growing mass and the development of cardiac arrhythmia. There are few cases associated with fibromas and cardiac haemangiomas.8,10,11 In cases of pericardial effusion associated with cardiac diverticula, it is believed to be caused by the rubbing of the diverticulum with the pericardium walls.
PERICARDIOCENTESIS
Definition
Fetal pericardiocentesis is the removal of fluid with a percutaneous catheter or needle from the pericardial sac. It is a safe and effective method to drain the effusion. It must be performed after 16 weeks of gestational age to avoid complications, just as with amniocentesis.
Indications
The aim of pericardiocentesis is to decompress the fetal chest, allowing the expansion of the lungs, reducing the pressure in the fetal venous system, causing a theoretical risk reduction for pulmonary hypoplasia and fetal hydrops.
Depending on the aetiology, for some cases a single procedure may resolve the condition. There have been reports in the literature of spontaneous resolution especially in idiopathic cases, but also in cases associated with cardiac diverticula.5,8,12,13 Since pericardiocentesis is considered a technique with a low risk to the fetus, it should be the treatment of choice in selected cases, such as large pericardial effusions that do not disappear or increase in a short period of time, to avoid the risk of pulmonary hypoplasia with severe sequelae to the fetus.12 For fetuses with moderate pericardial effusions outpatient follow-up should be done according to the underlying cause and fetal status, which may require weekly controls or controls every 48 hours.
In isolated pericardial effusion, the persistence of pericardial effusion is more important than the size. In some studies there were no correlations between the size of the pericardial effusion and the regression of pericardial fluid, adverse outcomes, or mortality rate.5,6,8 The pericardial effusion associated with cardiac tumours, usually teratomas, benefits from performing an intrauterine pericardiocentesis.9,14-16 The decompression of the pericardial cavity reduces the risk that the fetus may develop non-immune fetal hydrops. In these cases, intrauterine pericardiocentesis can help to reduce fetal hydrops with prolongation of pregnancy until lung maturity is reached, which prevents or delays the development of cardiac tamponade or pericardial effusion relapse. The use of pericardiocentesis immediately prior to delivery improves ventilation and haemodynamic stability in the preterm infant, prevents severe cardiorespiratory distress, facilitates neonatal stabilisation and intubation, and allows planned rather than emergency cardiothoracic surgery.15-18
It has been proposed that fetuses with cardiac tumours such as teratomas must be followed to assess the presence of fetal hydrops. In cases where hydrops develops and the fetus is >28–30 weeks and matured, delivery and postnatal intervention could be considered. In cases where there is insufficient lung maturity, pericardiocentesis can be performed to delay delivery for 1–2 weeks and allow time to mature the fetus before delivery. For fetuses <28 weeks old with severe pericardial effusion it is recommended to perform pericardiocentesis and if the effusion recurs, it is sometimes necessary to perform this procedure again16,19 and serial pericardiocentesis may be required to avoid prematurity with its related complications.12,20-23 Therefore, the aetiology associated with the pericardial effusion, gestational age, and fetal status should be taken into consideration to assess the need for fetal lung maturation with corticosteroids. We do not consider that fetal lung maturation is required in all cases <34 weeks of gestational age just because the procedure is being performed, but it should be considered in those cases where fetal status is compromised and could require an immediate delivery if fetal deterioration occurred. There have been reported cases in which a pericardial-amniotic shunt was placed and, in some cases, associated with pericardiocentesis. However, there are limited data available and further evaluation of its efficacy is needed.12,24
Technique
Fetal pericardiocentesis should be performed under sonographic guidance as an outpatient procedure. Two people are needed for the procedure: an operator to control the needle and transducer, and an assistant to help in aspiration of the fluid.
A fetal ultrasound should be performed initially to locate the most suitable place for puncture. The mother should be positioned as horizontally as possible, to allow better access to the amniotic cavity. Skin preparation with antiseptic solution and sterile field placement should be performed. The ultrasound probe should be prepared with an antiseptic solution prior to each procedure, since the probe is not covered with a sterile drape. We use a multi-frequency convex transducer with a 60×13 mm footprint and a 2–7 MHz bandwidth.
With a short 22-gauge needle, local anaesthesia is administered at the puncture site and with the local anaesthetic needle, a preliminary exploration to confirm the direction of the needle approach is made. Then, under direct visualisation a 20–22-gauge needle should be used to access the fetal chest. We recommend using a 20-gauge needle just as when performing amniocentesis. The puncture needle is gently inserted in the maternal skin and advanced through the uterus, amniotic cavity, and fetal chest under echographic guidance. Then, with a syringe, the fluid should be drained and finally the needle removed under echographic visualisation. The complete procedure must be performed with ultrasound guidance with continuous visualisation of the needle. Follow-up is performed every 48 hours during the first week, and after that the follow-up interval will be determined by the underlying condition that caused the effusion and fetal status.
If the woman is rhesus negative, anti-D immunoglobulin should be given within 72 hours after the procedure. If the amniotic fluid amount is too low to allow an adequate visualisation for the technique, an amnioinfusion is performed by administering saline through the puncture needle once the amniotic cavity has been reached.
Complications
To date, pericardiocentesis is a safe and effective procedure. The main complications are related to the amnion puncture, like with amniocentesis: temporary loss of amniotic fluid and premature rupture of membranes (PROM). Other rare complications are fetal injuries, vertical transmission of infectious diseases, intrauterine infection, pregnancy loss, and, rarely, septic shock.
REVIEW OF THE LITERATURE
We performed a PubMed search and reviewed the literature for cases with pericardiocentesis performed prenatally. Patients who underwent drainage by placing shunts additional to pericardiocentesis were excluded, and cases where only pericardiocentesis was performed as a pericardial effusion treatment were studied. Table 2 summarises the cases where pericardiocentesis was performed.
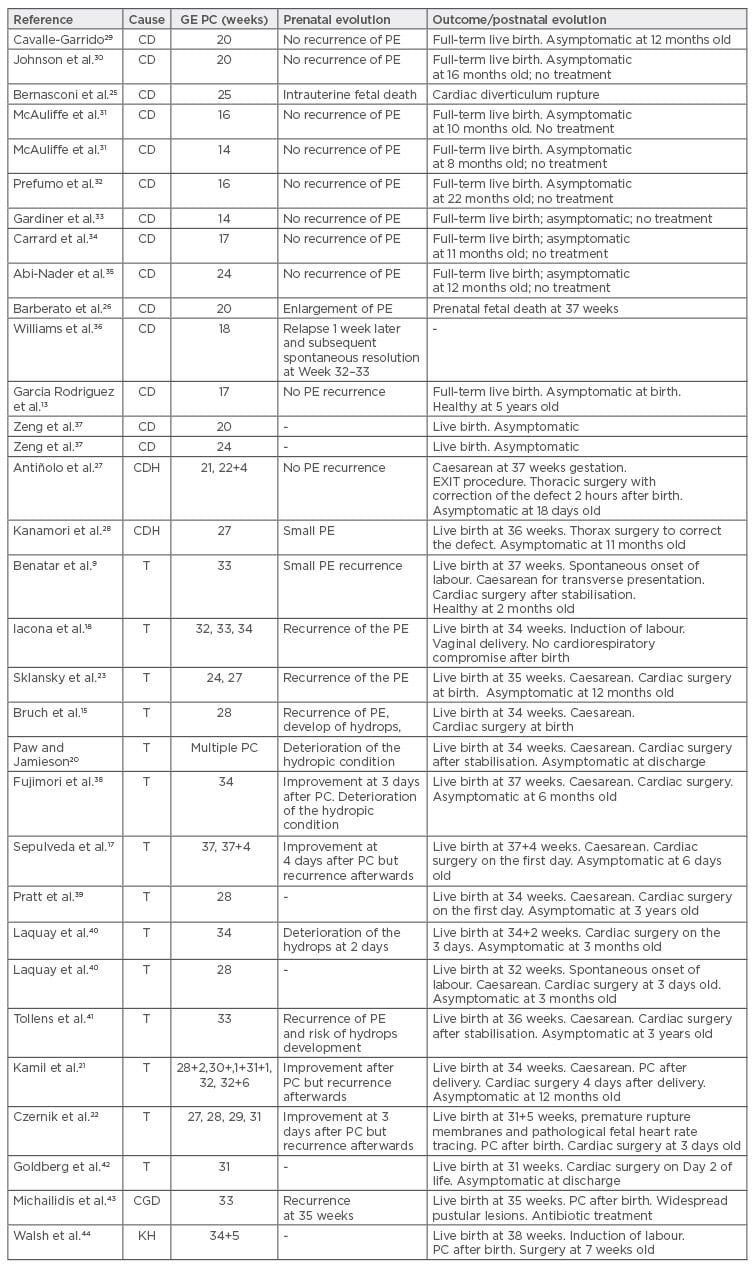
Table 2: Reported cases of fetal pericardiocentesis.
GE PC: gestational age of the pericardiocentesis; PC: pericardiocentesis; CD: cardiac diverticulum; CDH: congenital diaphragmatic hernia (Morgagni); T: cardiac teratoma; PE: pericardial effusion; CGD: chronic granulomatous disease; KH: kaposiform haemangioendothelioma.
At the time of writing, 33 cases of prenatal intrauterine pericardiocentesis have been reported. In 14 cases, pericardial effusion was associated with cardiac diverticulum, 14 cases to cardiac teratoma, 2 cases to congenital diaphragmatic hernia (intrapericardial hernia or Morgagni’s hernia), 1 case of kaposiform haemangioendothelioma, and 1 case of X-linked chronic granulomatous disease. In cardiac diverticula, the pericardial effusion was resolved with a simple pericardiocentesis. For cardiac teratomas eight cases used a single pericardiocentesis to drain the effusion and for six fetuses the use of serial pericardiocentesis was necessary.
There were no complications reported in relation to the pericardiocentesis, but in one case of cardiac teratoma, PROM occurred at 31 weeks. In this fetus a total of four pericardiocenteses were performed: three prior to PROM to improve fetal status associated with hydrops, extending in utero life for 4 weeks and allowing fetal lung maturation with corticosteroids at 29 weeks; and one pericardiocentesis performed prior to delivery at 31+5 weeks.22 Two fetal deaths were reported in association with cardiac diverticulum. In one case this was due to diverticulum rupture27 and in the other case it occurred 17 weeks after the procedure.33
Neonatal survival was high. All live neonates (n=31) were asymptomatic at hospital discharge. In the two cases of intrapericardial congenital diaphragmatic hernia (Morgagni) with pericardial effusion, the procedure avoided the development of pulmonary hypoplasia and avoided neonatal respiratory morbidity and fetal death.36,37 In conclusion, fetal pericardiocentesis is a safe procedure allowing the possibility of fetal lung maturation and improving fetal extraction with a better haemodynamic and respiratory condition at delivery.








