Abstract
Since its introduction into clinical practice, coronary angioplasty has seen game-changing advances incomparable to any other medical technology. In particular, the progression from balloon angioplasty to stent technology has not only seen significant advances in technology but, more importantly, improved patient outcomes.
The introduction of the drug-eluting stent (DES) has further pushed the technology to a new standard of care. However, even in the current day, top performing DESs still have several limitations. Their safety has been limited by suboptimal polymer biocompatibility, delayed stent re-endothelialisation, and local drug toxicity, leading to adverse clinical outcomes such as very late stent thrombosis, allergic reactions, and chronic inflammation. In addition, current DESs have a consistent yearly increase in late restenosis and revascularisations. Furthermore, durable polymer coatings used in first-generation DESs have been associated with mechanical complications and non-uniform coating, resulting in drug loss and poor distribution.
As a consequence, in recent years, the focus of research has been on the development of novel drug carrier systems including absorbable (or biodegradable) polymer coatings and non-polymeric stent surfaces. Additional improvements have included the development of more modern stent platforms. Optimised drug delivery requires a combination of refined stent designs and drug delivery technology. The combination of highly-refined bare-metal stent designs and polymer coating materials have been two areas of focus for the development and improvement of next-generation DESs. Despite all the changes in stent design and polymer materials, there has been little done in the past 15 years to improve drug elution profiles.
The need for new advancements in DES design to overcome late event occurrence, and possibly even improve on the clinical outcomes of current DESs, has led to interest in moving away from the standard drug elution profile to explore alternatives. A new manufacturing technique that may have overcome traditional limitations has led to the development of a novel stent platform. This review will explore the principles, device technology, and clinical data to date on a crystalline form of the anti-restenotic drug for stent implantation. This approach could truly be game changing due to an improved elution pharmacokinetic profile, as well as reduced local toxicity and improved long-term biologic arterial wall response.
INTRODUCTION
Coronary artery disease (CAD) is the most common type of heart disease and the first cause of mortality worldwide in people aged ≥60 years.1 Complications include acute coronary syndromes (ACS) (unstable angina or myocardial infarction [MI]), congestive heart failure, cardiac arrhythmias, and sudden death.
Since its introduction into clinical practice, coronary angioplasty has witnessed a remarkable surge in innovation incomparable to any other medical technology. In particular, stent technology has been a source of remarkable improvement, reaching an outstanding rate of development which has nowadays increased in a similar fashion to that of Moore’s Law (pertaining to computing hardware, the number of transistors in a dense integrated circuit doubles approximately every 2 years).
WIDESPREAD UTILISATION OF STENTS IN CORONARY ARTERY DISEASE
Bare-Metal Stenting
Wide acceptance of coronary stenting was based on the results of the BElgian NEtherlands STENT (BENESTENT)2 and the STent REStenosis Study (STRESS)3,4 trials, which showed the superiority of bare-metal stenting over plain old balloon angioplasty (POBA) (also called percutaneous transluminal coronary angioplasty) in terms of reduction of angiographic restenosis and need for repeated intervention in focal lesions and large coronary arteries.2,5-7
Since then, the growing use of stents in ever more complex lesions and patients8,9 has stimulated the introduction of a rapidly increasing number of different stent design characteristics and catheter technology advances (rapid-exchange, flexible tips, increased retention, decreased dislodgement, etc.). Among the reasons why different stent characteristics and designs have been proposed is the concept of physiological mechanisms influencing outcomes; indeed, a primary concern of stent development is the need to reduce device profile and to increase flexibility to facilitate safer delivery and improve clinical performance.
In addition to the improvements in bare-metal stent (BMS) design, another clinical advance was the introduction of oral dual antiplatelet therapy (DAPT). Antiplatelet agents have almost eliminated the occurrence of acute and subacute stent thrombosis (ST), hours to weeks after stent deployment. ST can lead to significant clinical events (sudden cardiac death or acute MI).
Other important issues that have been improved upon with better stent designs are lesion coverage to minimise plaque prolapse; increasing radial support to prevent elastic recoil of the artery; and greater radiological visibility to precisely position stents. Furthermore, the possibility of easy access to side branches through the stent struts of a deployed stent in bifurcation lesions has become progressively more important.
First-generation stenting devices were made of stainless steel (BMS), and aimed to improve arterial restenosis over POBA. Still, 1-year post-procedure, these devices were associated with a residual risk of clinical restenosis as high as 20%, requiring target-lesion revascularisation (TLR).2,6,10 The BMS design continued to advance with the use of alloy metals, allowing for thinner struts and greater flexibility while maintaining radial strength.
The Drug-Eluting Stent Revolution Starts
The high incidence of restenosis and the need for TLR, as well as further improving clinical benefit, were the stimuli for developing the drug-eluting stent (DES). The first DES had a polymer coating that served as the vehicle for delivery of an anti-restenotic drug, (e.g. the Cypher™ sirolimus-eluting stent [SES] and Taxus™ paclitaxel-eluting stent [PES] devices), which is slowly delivered over a few months following implantation. The first few generations of DES used a permanent or durable polymer coating that remained on the stent indefinitely, even though the entire drug was released from the polymer within a few months of stent deployment. The introduction of DESs further pushed the technology to a new level of patient care, through a combination of the increased understanding of the biology of restenosis, the selection of drugs that target one or more pathways in the restenotic process, controlled-release drug delivery strategies, and the use of the stent as a delivery platform.
Although first-generation DESs were demonstrated to be superior to BMSs with regards to the rates of recurrent MI, ST, and restenosis/TLR, their safety has been limited by suboptimal polymer biocompatibility, delayed stent re-endothelialisation contributing to late and very-late ST, initial spike or drug dumping, and local drug toxicity.11-21 Furthermore, durable polymer coatings used in first-generation DESs have been associated with mechanical complications (e.g. polymer delamination, weaved polymer surface leading to stent expansion) and non-uniform coating resulting in non-uniform drug distribution.22
Second-generation DESs including Xience™ (Abbott Vascular, IL, USA), an everolimus-releasing durable polymer cobalt-chromium (CoCr) DES which became the gold standard; Promus™ (Boston Scientific, Boston, MA, USA), an everolimus- eluting stent (EES); and Resolute™ (Medtronic Cardiovascular, Minneapolis, MN, USA), a zotarolimus-eluting stent (ZES) used a durable polymer coating that was more biocompatible, resulting in better re-endothelialisation and less inflammation but still carried the problem of an increasing rate of late and very-late restenosis and TLR.
Durable Polymer Coating Drug-Eluting Stents
As the stent is a foreign body not recognised by the blood, the most threatening acute complication of a stenting procedure is ST. This event has been reduced to <1–2% (compared to 5–7% in the first trials), due to the introduction of high-pressure deployment of the stent23-25 and the use of DAPTs (aspirin and a thienopyridine), instead of aspirin and an oral anticoagulant.24 It has been demonstrated that there are substantial differences in haemodynamic and wall rheological characteristics of implanted stents of different designs, and the ‘hydrodynamic compatibility’ of a stent is now recognised as an important feature of ideal stent design. In this setting, Gurbel et al.26 recently demonstrated that stent design can also affect the degree of platelet activation; thus, ST may be higher with coil than tubular stents.
Moreover, after the initial vessel trauma with intimal and medial injury, permanent polymer coatings can generate focal chronic inflammation with cell infiltration and extracellular matrix remodelling, leading to neoatherosclerosis formation, a contributor to late (and very-late) post-procedure restenosis and/or ST, as demonstrated in animal and human pathological studies.27-33 Therefore, the cardiology community has focussed its attention on safety, with the introduction of a stricter indication of DES use and prolonged DAPT (European Society of Cardiology [ESC]/European Association for Cardio-Thoracic Surgery [EACTS] guidelines recommend DAPT for 6–12 months with 162–325 mg acetylsalicylic acid plus a platelet P2Y12 receptor inhibitor).11,26,34-40
The optimal duration of DAPT is, however, a controversial topic that has been debated in recent years.41,42 Recently, Verdoia et al.34 published a meta-analysis of 11 randomised trials, which suggested that 3–6 months of DAPT after second-generation DESs provided a reduction in mortality and major bleeding risk, but at the cost of a slightly increased risk of MI and ST; nevertheless, it was evaluated as the safest duration. Major concerns still remain with the observation of a higher incidence of very late ST (i.e. ST that occurs >12 months) in patients receiving a DES versus patients receiving a BMS.27-33 Issues related to the increased incidence of very-late ST are late malapposition, incomplete strut coverage, and chronic inflammation. The mechanisms behind late ST seem to be multifactorial; factors including inappropriate stent deployment techniques and delayed or inadequate stent surface endothelialisation have been implicated.43-46
The challenge of ST could be addressed by applying an antiproliferative drug to the vascular smooth muscle cells activated by stenting and subsequent inflammation, rather than to the endothelial cells, which must proliferate. To that effect, the abluminal coating with sirolimus derivatives, which leaves the luminal side of the stent free from the drug and polymers, has been introduced in some of the latest DESs together with bioabsorbable polymers, e.g. poly(acid), as polymers could potentially cause chronic inflammation and subsequent endothelial dysfunction.28,47,48
The abluminal coating was designed to enhance re-endothelialisation, i.e. proliferation of endothelial cells, on the luminal surface of the stent, by preventing exposure of this surface to the antiproliferative drug. Accordingly, reduced late ST and better endothelial function of the DES-implanted artery employing the abluminal coating with sirolimus derivatives have been reported in both preclinical and clinical settings.49-52 On the contrary, no significant differences in endothelialisation between the abluminal coating and the conventional full-surface coating of DESs have been reported.53,54
Recently, the results of the ODESSA trial confirmed this expectation. Among three different DESs; the Cypher (SES), Taxus (PES), and Endeavor® (ZES); and the Liberté BMS, the ODESSA Trial observed the rate of uncovered struts and late malapposition after 6 months with optical coherence tomography (OCT). The results demonstrated a difference in the rate of uncovered struts and malapposed segments among different DESs and among DESs versus BMS (8.2%, 4.3%, 0.02%, and 0.9% for Cypher, Taxus, Endeavor, and BMS, respectively).55
Further Improved Safety: Thin Struts, Absorbable Polymers, Polymer Free, Bioresorbable Stents
In recent years, the focus of clinical research has not been limited to the composition, distribution, and thickness of the polymers. Improvement strategies have also focussed on the material, geometry, and thickness of the stent platforms, as well as the selection and dosage of antiproliferative agents. Stent platforms consisting of cobalt or CoCr instead of stainless steel allowed stent strut thickness to be reduced by more than half as compared with early-generation devices, all the while maintaining radial force and stent visibility. Thin-strutted BMSs have been associated with a reduced risk of restenosis.56 Moreover, experimental data indicate a lower thrombogenicity, which may be related to more rapid endothelialisation compared with thick-strutted stent types.57 Antiproliferative substances of the rapamycin family prevailed over paclitaxel in new-generation DESs and brought forth several limus analogues with comparable efficacy.58
Of note, the permanent polymers used in early-generation SES (polyethylene co-vinyl acetate/poly-n-butyl methacrylate) were modified with new-generation permanent polymer EESs (poly-n-butyl methacrylate/co-polymer of vinylidene fluoride and hexafluoropropylene). The combined effects of technological progress on different levels has translated into improved clinical outcomes, with elimination of previous concerns over very-late ST, while the anti-restenotic efficacy of early- generation DESs has been preserved: this constitutes the current standard of care. As a consequence, in recent years the focus of clinical research has been on the development of novel drug carrier systems, including thinner strut structures, absorbable (or biodegradable) polymer coatings and nonpolymeric stent surfaces.
The clinical need for bioresorbable or biodegradable DES devices was based on the fact that vessel scaffolding is only needed transiently.59,60 The perspective of positive long-term outcomes for patients with no residual scaffold seemed appealing and led to the development of alternative fully bioresorbable devices such as the Absorb™ vascular scaffold (Absorb BVS, Abbott Vascular), a first-of-its-kind EES that is naturally resorbed and fully metabolised.61-65 Additional improvements included the development of more modern platforms (e.g. better deliverability, radiopacity, flexibility, and radial strength) as well as the use of novel antiproliferative agents or reduced doses of currently approved antiproliferative drugs (Table 1).
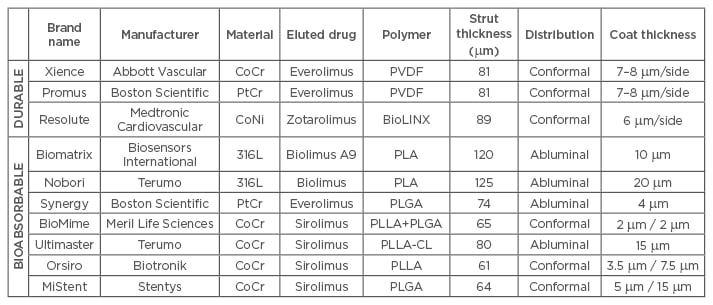
Table 1: Polymer type and stent strut thickness among different polymer coated drug-eluting stents (durable versus bioabsorbable) of second and third-generation.
316L: 316L stainless steel; BioLINX: C10, C19, and polyvinylpyrrolidone; CoCr: cobalt chromium; CoNi: cobalt nickel; PLA: poly-D/L-lactic acid; PLGA: polylactic-co-glycolic acid; PLLA: poly(L-lactide); PLLA-CL: poly(L-lactide-co-caprolactone); PVDF: poly(vinylidene fluoride); PtCr: platinum chromium.
The Drug-Eluting Stents Paradigm
Optimised drug delivery requires a combination of effective drug delivery technology and refined stent design. Stent-based drug delivery has been accomplished by three distinct mechanisms:
a) Bioabsorbable polymeric stents can be loaded with a drug that is eluted slowly over time
b) Metal stents can have a drug bound to their surface or embedded within macroscopic fenestrations or microscopic nanopores, thus providing more rapid drug delivery
c) Metal stents coated with an outer layer of polymer (bio-stable or bioabsorbable) can be drug-loaded; in turn, drug coating could be conformal (around the stent strut) or abluminal (only towards the arterial wall), thus providing more controlled and sustained drug delivery, which might allow more effective drug-tissue interactions
Moreover, the combination of highly refined stent designs and polymer coating materials has been the standard approach in several DES initiatives. In this regard, the novel combination of a biodegradable polymer with a thin-strut CoCr or platinum-chromium platform introduces a logical next step in the refinement of biodegradable polymer DES.
Limitations of Absorbable Polymer Coated Drug-Eluting Stents with Conventional (Amorphous) Drug Elution
The need for new DES advancements in percutaneous coronary interventions (PCI) to overcome late event occurrence, like late restenosis, stems from the fact that current drug elution profiles are a limitation of DES engineering characteristics.64,66-68
The latest advances in DES technology have been mainly focussed on stent material, strut thickness, or polymer development because, until recently, technological limitations prevented the optimisation of the drug elution profile. Most manufacturing techniques for DES production involve the use of solutes to apply the drug to the polymer/stent, leaving the drug in an amorphous or aqueous form. This process limits the margin of control over the elution profile, which is mainly dependent on diffusion of the drug along a concentration gradient. As a consequence, the distribution profile of the drug consists of an uncontrolled initial burst, which in turn may contribute to delayed healing and possibly late adverse events.69-71
This rapid drug burst quickly overwhelms smooth muscle cell receptors, followed by a rapid clearing of the drug due to systemic loss. Therefore, there is a limited therapeutic benefit for the amount of drug released in this initial burst. Because the polymer is the vehicle for the drug release, the polymer is required to elute the remainder of the drug. At a certain point the tissue drug level will drop beneath the therapeutic level and have no effect. However, the polymer will continue to reside for an extended period of time, which leaves the vessel vulnerable to its negative effects.
Moreover, the drug elution profile of conventional DES decays with a logarithmic-type pattern, and is relatively short. Lengthening the elution period would require an increase in the total drug load, with a significant increase of the initial burst of the drug. This may reach toxic levels,69-72 which may in turn promote delayed strut coverage, consequently increasing late and very-late ST with necessity of extended DAPT.
ABSORBABLE POLYMER IN LATEST GENERATION DRUG-ELUTING STENTS: IS THERE ROOM TO IMPROVE CLINICAL OUTCOMES?
Crystalline Drug Form Breaks the Relationship Between Polymer Presence and Drug Elution
A new manufacturing technique seems to have overcome this limitation and led to the development of a novel system. The MiStent SES Sirolimus Eluting Absorbable Polymer Coronary Stent System (Stentys/Micell Technologies, Durham, NC, USA) is a balloon-expandable stent that received Conformité-Européenne (CE)-marking in 2010 for the treatment of CAD. MiStent SES is a CoCr alloy stent platform with very thin 64 m struts (one of the thinner struts currently present in the European market), based on the Eurocor (CE-marked) Genius MAGIC® CoCr Coronary Stent System. The main innovation of MiStent SES is that this device includes a coating made of polylactide-co-glycolic acid (PLGA; a fully bioabsorbable polymer) delivering sirolimus in a crystalline form. A patented, supercritical fluid technology allows a rigorously controlled drug and polymer coating to be applied to this BMS.
Possible Advantages of MiStent Sirolimus-Eluting Stents in Terms of Efficacy and Safety Outcomes
Optimising the Drug Elution Profile: Fast Polymer Absorption with Sustained Slow Drug Release
The innovative coating technology allows for encapsulation of sirolimus as tiny crystals. This crystalline form is responsible for a precise and consistent drug elution profile: as the polymer softens and disperses from the stent struts into the adjacent tissue, the crystals are slowly released and deposited in the tissue surrounding the stent (Figure 1). The rate of elution is independent of the polymer coating. Rather, it is dependent on the dissolution of the crystal and then diffusion along a gradient, eliminating the initial uncontrolled burst of the drug observed in other DESs.
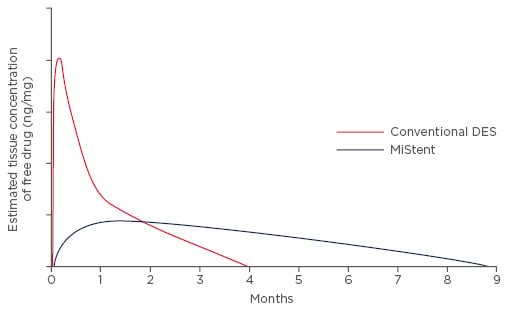
Figure 1: Crystalline sirolimus allows for a gradual, linear, and long-term elution profile targeted to minimise the long-term hyperplastic response of the vessel.
Stentys, data on file.
DES: drug-eluting stent.
The drug elution profile is gradual, near linear, and much longer compared with other DESs. The controlled and sustained release of therapeutic concentrations of the drug provides a continued local antiproliferative and anti-inflammatory effect for several months, thus reducing vessel over- scarring and inflammation. In this respect, data from the DESSOLVE II Study demonstrate that vessel healing is similar to that obtained with other contemporary DESs, with no paradoxical vasoconstriction effect after acetylcholine injection.73 Moreover, in this study, the OCT control subgroup reported an almost complete percentage of strut coverage as early as 4 months after MiStent implantation, thus suggesting an optimal re-endothelialisation rate and absence of toxicity due to the presence of a high initial burst dose of drug, as seen with earlier generation DESs.
According to preclinical studies, the PLGA/sirolimus coating is cleared from the stent in 45–60 days, and absorbed into the tissue within 90 days, leaving a BMS.74 PLGA is completely absorbed into the surrounding tissue within 3 months of implantation to promote fast vessel healing, long-term patency, and compatibility with the artery, while allowing for drug elution to continue beyond its absorption. In addition, the fast polymer absorption characteristic limits polymer exposure duration, thereby reducing some safety risks associated with currently available DES technologies.
Crystalline sirolimus remains in the tissue and continues to elute and maintain therapeutic levels of the drug into the surrounding tissue for up to 9 months. This long-term delivery of the drug is a unique feature which inhibits vessel restenosis.74,75 Preclinical and early clinical data seem to suggest that this controlled elution profile may be beneficial over the long-term and translate into clinical benefits by slowing the progression of late lumen loss (LLL) and preventing the TLR catch-up phenomena.74,75
Safety
The MiStent SES may also offer a safety benefit, as the polymer absorption occurs within 3 months thereby limiting the duration of polymer exposure, and therapeutic levels of sirolimus persist beyond the presence of polymer, further limiting inflammation. This characteristic is not currently available in other DESs and should increase the safety profile of such stents even with short DAPT. However, this hypothesis should be proven in powered randomised clinical trials.
CLINICAL EXPERIENCE ON MISTENT SIROLIMUS-ELUTING STENTS
DESSOLVE I and DESSOLVE II Clinical Trials
In the DESSOLVE I and II Trials, patients with discrete de novo lesions in native coronary arteries were enrolled and followed for clinical events at predefined intervals. The 4-year clinical follow-up from these two studies were recently presented and demonstrate the long-term efficacy and safety of this device. All available patients are currently undergoing long-term follow-up.
DESSOLVE I
The DESSOLVE I Trial was the first clinical evaluation of the safety and efficacy of the MiStent SES, and was conducted in 30 patients with lesions ranging from 2.5–3.5 mm in diameter and amenable to treatment with a ≤23 mm long stent. Subjects were enrolled across five study centres in New Zealand, Australia, and Belgium.76 Three independent subgroups of 10 patients were each evaluated using angiography, intravascular ultrasound (IVUS), and OCT at three time points post-treatment: 4, 6, and 8 months with all available patients returning at 18 months. The primary efficacy endpoint was in-stent LLL. Safety was assessed by incidence of major adverse cardiovascular events (MACE) and presence of strut coverage with tissue within the treated artery at each time point.
At 4, 6, 8, and 18 months of follow-up, the MiStent SES was associated with a low and stable in-stent LLL (4 months, median 0.03 mm, range: -0.22–0.21 mm; 6 months, median 0.10 mm, range: -0.03–1.20 mm; 8 months, median 0.08 mm, range: -0.01–0.28 mm; 18 months, median 0.08 mm, range: -0.30–0.46 mm). In all 30 patients, the small mean±SD in-stent LLL up to 8 months of 0.1±0.2 mm (95% confidence interval: 0.04–0.5 mm), demonstrated stent efficacy.
At OCT examination, the proportion of uncovered stent struts decreased from a median of 7.3% (range: 0.4–46.3%) at 4 months to 0% (range: 0.0–3.4%) at 18 months. The percentage of neointimal volume obstruction as evaluated by IVUS increased from a median of 5.3% to 9.1% between 4 and 6 months and remained nearly unchanged thereafter through to 18 months. An evaluation of the safety outcomes at 3 years was published for DESSOLVE I. At this time point, there were no target-vessel MACE for MiStent SESs. Two non-target-vessel non-Q-wave MIs were reported. In addition, there was no evidence of ST or TLR.77,78
At the 27th Annual Transcatheter Cardiovascular Therapeutics (TCT) Conference held in San Francisco in October 2015, 4-year safety outcomes were consistent with previous clinical findings, with no target-lesion failure (TLF), no target-vessel MACE, and no ST. Two non-target-vessel non-Q-wave MIs were reported as before, along with one target-vessel revascularisation (TVR), non-TLR at 1,280 days.79,80
DESSOLVE II
The DESSOLVE II CE-mark Trial was a randomised (2:1), multicentre, superiority study conducted in 184 patients from 26 sites in Europe and New Zealand, with documented stable or unstable angina pectoris.73 The primary endpoint was superiority of the MiStent SES in minimising in-stent LLL at 9 months, versus Medtronic’s Endeavor Sprint DES (delivering zotarolimus). LLL was measured by an independent angiography core laboratory in de novo coronary lesions from vessels ranging from 2.5–3.5 mm in diameter and amenable to treatment with a ≤30 mm long stent.
DESSOLVE II met all study objectives, as the MiStent SES demonstrated a competitive in-stent LLL, with a mean in-stent LLL of 0.27±0.46 mm in 103 MiStent SES patients versus 0.58±0.41 mm in 52 Endeavor patients (p<0.001, Figure 2). The MiStent SES achieved a strong safety signal, as the proportion of uncovered stent struts by OCT at 9 months was very low in both groups (MiStent, 0.3% versus Endeavor, 0.0%; Figure 3). The mean neointimal thickness of covered struts (p=0.002) and percent net volume obstruction (p≤0.003) were significantly lower in the MiStent SES group than in the Endeavor group. MACE and ST rates were low and comparable between groups.73
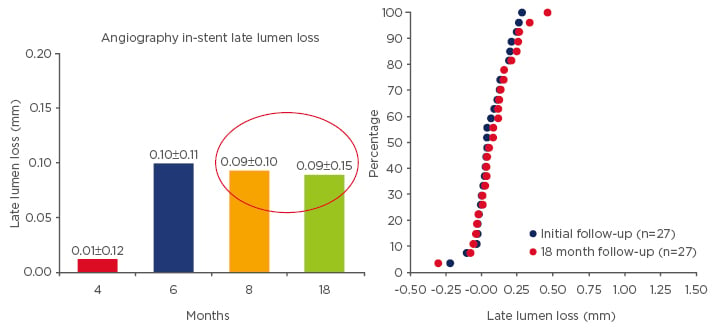
Figure 2: Cummulative distribution curves of the In-stent late lumen loss for MiStent between matched cases during study course.88,89
Data represents matched cases for each time point with serial 3D analysis.
4 months, n=9; 6 months, n=9; 8 months, n=9; 18 months, n=27.
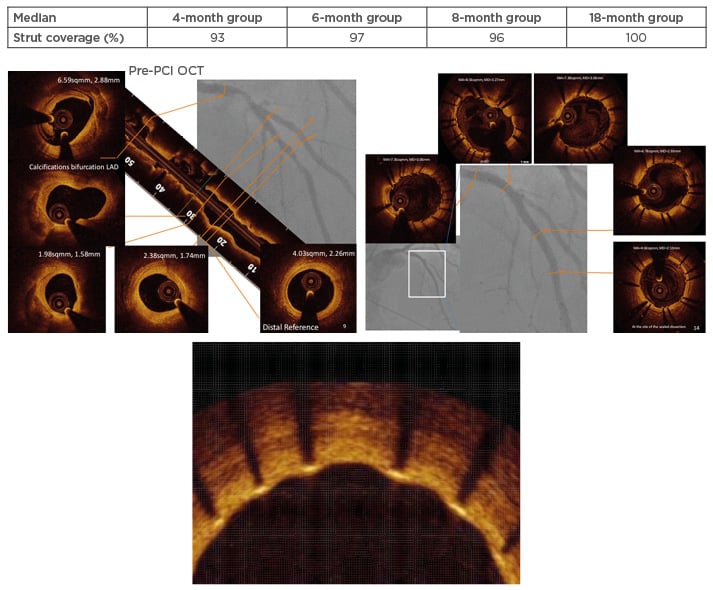
Figure 3: Case example from the DESSOLVE II study.76,88
Table indicates the median percentage of strut coverage during the study time intervals. Figure shows the OCT pre-PCI (left panel) with a complex plaque in the proximal and middle segment of the LAD, which was stented with two MiStents in overlapping (right panel). OCT result at 6 months is shown in the lower magnified insert.
LAD: left anterior descending artery; OCT: optical coherence tomography; PCI: percutaneous coronary intervention.
Safety outcomes at 2 years were subsequently published and further supported clinical implementation with low event rates, as evidenced by a 6.7% MACE rate for the MiStent SES versus 11.7% for Endeavor (p=0.129).77,81 There was no definite or probable ST for the MiStent SES (versus 1.7% with Endeavor), while the TLR rate was identical with both devices (1.7%). At 3 years, rates of MACE for MiStent and Endeavor were 8.3% versus 15.3% (p=0.197), respectively; TLR and MI rates for the MiStent SES and Endeavor stent were 2.5% and 3.4% (p=0.665) and 3.3% and 5.1% (p=0.686), respectively.82
The updated 4-year results of DESSOLVE II presented by Dr Alexandra Lansky at TCT 2015 confirmed the safety profile of the MiStent SES with low event rates, as evidenced by a 13.3% MACE rate for MiStent SES (n=118) versus 20.3% for Endeavor (n=59), and this difference was mainly driven by TVR rates (4.3% versus 10.2% for MiStent SES and Endeavor, respectively). There was no definite/probable ST for the MiStent SES (1.7% with Endeavor).80
Retrospective Cross-Study Propensity Analysis
In a late-breaking clinical trial session presented at the 2015 European Association of Percutaneous Cardiovascular Interventions (EuroPCR) congress, Dr Lansky presented the results from a retrospective cross-study propensity analysis comparing the MiStent SES to Abbott’s Xience V stent, the market leader.83 The results were subsequently published in the American Journal of Cardiology in February 2016.84 The objective of this study was to compare the clinical outcomes of both devices at 1 and 3 years post-implantation. A retrospective, patient level, propensity-matched analysis was conducted comparing data from the DESSOLVE I and II studies to the Xience V arm of the ISAR-TEST-4 study with pre-specified baseline and lesion characteristics. As all three studies used very similar event definitions, clinical and angiographic endpoints were compared between treatment groups in the matched cohort.
The primary endpoint was TLF at 1 and 3 years. More than 800 patients (MiStent, n=153 and Xience, n=652) were included, with propensity matching in 204 patients (MiStent and Xience, n=102 each). The analysis demonstrated statistically significant reduced TLR rates at 1 and 3 years for the MiStent SES, as compared with the Xience V DES (Table 2).
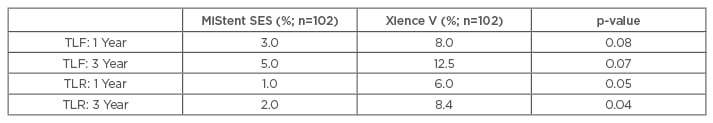
Table 2: Propensity-matched analysis between the MiStent sirolimus-eluting stent and the Xience V devices.83,84
TLF: target-lesion failure; TLR: target-lesion revascularisation; SES: sirolimus eluting stent.
At 3 years, there was no significant difference in target-vessel MI (MiStent 2.0% versus Xience 3.1%, p=0.34), and there were no additional late or very-late ST in either group. These results suggest that the improved device design characteristics translated into clinical benefits in the targeted patient populations.
CURRENTLY ONGOING STUDIES
DESSOLVE III Post-Market Clinical Trial
As the DESSOLVE II Study was not powered to compare clinical outcomes between the MiStent SES and Endeavor, a larger study was initiated. DESSOLVE III is a landmark prospective, balanced, randomised, controlled, single-blind, multicentre clinical study comparing MiStent SES to Xience.85 Patient enrolment (1,400 ‘real-world all-comers’ patients from 20 hospitals throughout Europe) was completed in December 2015, with early results expected in the first half of 2017.86 Patients in the trial suffered from symptomatic CAD, including those with chronic stable angina, silent ischaemia, or ACS, and qualified for PCI.
The primary endpoint is a non-inferiority comparison of a device-oriented composite endpoint or TLF of the MiStent SES group versus the Xience group at 12 months post-procedure. This trial will also include a sub-study on the OCT evaluation of 60 patients (30 assigned to each treatment group), in order to explore volume obstruction and frequency of neoatheroma formation over time (assessments at 6 and 24 months post-treatment).
DESSOLVE C Clinical Trial
DESSOLVE C is a prospective, randomised, controlled, single blind, multicentre clinical trial comparing the MiStent SES to the Tivoli™ stent. The study is currently enrolling in China at approximately 18 clinical sites. The enrolment is expected to include approximately 428 patients with symptomatic CAD, including those with chronic stable angina, silent ischaemia, and ACS who qualify for PCI.87 Patients will be randomised to the MiStent SES or the Tivoli® device (a bioabsorbable polymer-based SES [Essen Technology Company, Beijing, China]). The primary endpoints will be the 9-month in-stent angiographic LLL along with 12-month clinical outcomes between both devices.
CONCLUSIONS
Numerous new and exciting trends are leading to a variety of improved DES platforms. The ideal DES has not been determined, but will most likely incorporate a number of new and improved materials and delivery mechanisms to further enhance safety, efficacy, and cost efficiency. Among those characteristics, the development of crystalline forms of anti-restenotic drugs combined with a thin strut BMS and fast absorbing polymer coating for stent implantation could truly be game changing due to improved elution pharmacokinetic profile, decreased local toxicity, and improved long-term biologic arterial wall response.
In this setting, the MiStent SES could be the driver for a new class of DES associated with meaningful clinical and safety outcomes, building on the successive revolutions and innovations in PCI over the last four decades.








