Abstract
Introduction: In November 2016, surgical antibiotic prophylaxis (SAP) guidelines for cardiothoracic surgeries at the authors’ centre were updated. SAP was reduced from 48 to 24 hours, and dual cover with vancomycin and cefazolin instead of vancomycin monotherapy was recommended for patients colonised with methicillin-resistant Staphylococcus aureus. This study was conducted to review compliance to the updated guidelines, and compare the incidence of surgical site infections (SSI).
Methods: A list of patients undergoing sternotomy in National Heart Centre, Singapore, from March 2016 to February 2019 was extracted from the hospital’s electronic database; every fourth patient was included in the analysis. The patients were divided into three groups: Group 1 (before guideline revision, March–October 2016), Group 2 (post-guideline implementation, July 2017–May 2018), and Group 3 (July 2018–February 2019). Compliance to guidelines, incidence, and epidemiology of SSIs within 90 days of surgery were evaluated.
Results: 509 patients (Group 1: 149; Group 2: 184; Group 3: 176) were included. There was appropriate selection and timely administration of SAP across all three groups. Post-guideline implementation, the proportion of patients on SAP for >24 hours decreased from 149 (100%) in Group 1 to 55 (29.9%), and 67 (38.1%) in Group 2 and 3, respectively (p <0.001). Despite the reduction in SAP duration, SSI rates remained stable: 4.7%, 3.3%, and 5.1% in Group 1, 2, and 3, respectively (p=0.662).
Conclusion: Guideline implementation significantly reduced SAP duration in the authors’ cardiothoracic surgeries, with no increase in SSIs. Continual feedback to ensure sustained compliance may be necessary.
INTRODUCTION
Sternal wound infections post-cardiothoracic surgeries are associated with increased morbidity and mortality.1,2 More than half are due to Staphylococcus aureus or coagulase-negative Staphylococcus epidermidis. Yet, there is limited evidence for optimal choice (monotherapy versus combination therapy) and duration of surgical antibiotic prophylaxis (SAP) in cardiothoracic surgeries for the prevention of sternal wound infections.
Most guidelines support the use of a first-generation cephalosporin (e.g., cefazolin) for perioperative prophylaxis of sternotomy, and in patients with β-lactam allergy, vancomycin.1-3 In institutions with high incidence of methicillin-resistant S. aureus (MRSA), vancomycin monotherapy is often used as first-line prophylaxis. However, β-lactams may have superior activity against methicillin-susceptible S. aureus (MSSA) compared to vancomycin.4 For example, Finkelstein et al.5 showed that MSSA surgical site infections were more common in patients receiving vancomycin monotherapy for cardiothoracic surgery. Therefore, combination antibiotic therapy with vancomycin and cefazolin have been used for perioperative prophylaxis in patients who are at risk of MRSA infections (e.g., patients colonised with MRSA undergoing sternotomies), with vancomycin limited to one or two doses2,6-8 to mitigate the risk of acute kidney injury associated with the concurrent use of β-lactams and vancomycin.6,9
Another area of controversy pertains to the duration of cardiothoracic surgical prophylaxis. Surgical prophylaxis durations are often extended in clinical practice, despite recommendations from the Society of Thoracic Surgeons, American College of Cardiology/American Heart Association, as well as the American Society of Health-System pharmacists to limit the duration from 24 to 48 hours.3 Administering antibiotic prophylaxis beyond 48 hours may have no additional benefit, but it may result in the development of infections with drug-resistant organisms.10,11 In contrast, the comparative data to show whether perioperative prophylaxis for 24 hours is as effective and safe as 48 hours is scarce.1-3,10 The recommended duration of prophylaxis for 24–48 hours is based on expert opinion.
In November 2016, the Antimicrobial Stewardship Unit in Singapore General Hospital (SGH), Singapore, collaborated with the Department of Cardiothoracic Surgery in National Heart Centre Singapore (NHCS), Singapore, in updating the antibiotic prophylaxis guidelines for cardiothoracic surgeries (Table 1). The most significant change in the guideline was the reduction in the duration of surgical antibiotic prophylaxis from 48 to 24 hours. In addition, for patients who were colonised with MRSA, there was an added recommendation for both vancomycin and cefazolin to be administered,6-8 as there was an increasing trend of MSSA sternal wound infections in patients on vancomycin-only prophylaxis from routine surveillance (based on the authors’ local unpublished data).
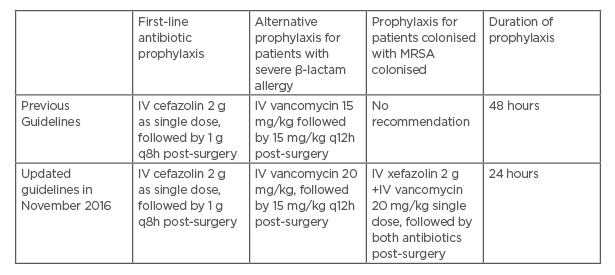
Table 1: Cardiothoracic surgery antibiotic prophylaxis guidelines in Singapore General Hospital and National Heart Centre Singapore.
*MRSA decolonisation was performed for all patients who are MRSA-positive prior to surgery pre and post-guideline implementation.
Note: No local antibiotic prophylaxis was administered, as this is not a routine practice in this institution.
IV: intravenous; MRSA: methicillin-resistant Staphylococcus aureus; q8h: every 8 hours; q12h: ever 12 hours.
The authors reviewed the impact of the updated SAP guidelines pre- and post-implementation, as described below.
METHODS
Study Design
This was a retrospective single-centre study, conducted as a quality improvement project to primarily evaluate the surgeons’ compliance to SAP for cardiothoracic surgeries involving sternotomies, and compare the incidence and epidemiology of surgical site infections (SSI) after shortening perioperative prophylaxis from 48 to 24 hours as part of the secondary objective. A waiver of informed consent was obtained from SingHealth Centralised Institutional Review Board (CIRB).
Pre-guideline Implementation
The SGH antimicrobial stewardship unit reviewed international recommendations on SAP in cardiothoracic surgery, as well as the authors’ hospital data on the incidence and epidemiology of post-surgical sternal wound infections. These findings, and the authors’ proposed SAP guideline updates (Table 1) were shared with the cardiothoracic surgeons, who then accepted the changes. To improve compliance to the updated guidelines, education roadshows with the cardiothoracic surgery department were conducted and the anaesthesiology department was also informed of the changes. Order sets in the electronic prescribing system were also created concurrently for ease of physician prescription. The guidelines were finally implemented in November 2016.
Post-guideline Evaluation and Data Collection
A list of all patients (above 18 years old) undergoing cardiothoracic surgery with sternotomy in SGH/NHCS from March 2016 to February 2019 was extracted from the hospital’s electronic database. As this study was done to quickly assess the outcome of the interventions and to provide timely feedback to surgeons, the authors opted to systematically sample every fourth patient in the list, and include only these patients in the analysis. The patients were then divided into three groups: Group 1 (patients admitted between March–October 2016, prior to guideline updates), Group 2 (patients admitted between July 2017–May 2018, after the implementation of the revised guidelines), and Group 3 (patients admitted between June 2018–February 2019, to assess persistence of guideline compliance). Even though the updated guidelines were implemented in November 2016, compliance to guidelines was only evaluated from July 2017, to factor time for guideline adoption.
Patient demographics, MRSA colonisation status, drug allergy, antibiotic administration records pre- and post-surgery, and clinical documentation of SSIs, as well as microbiological data from surgical site specimens collected within 90 days of surgery were retrospectively extracted from electronic health records, and recorded in a standardised data collection form.
Primary Objective
Compliance to guidelines in regard to choice of antibiotics, duration of prophylaxis, and timing of antibiotic administration were assessed for all three groups. Choice of antibiotic prophylaxis and duration of prophylaxis was deemed appropriate if they were in line with guideline recommendations as outlined in Table 1. Timing of antibiotic administration before surgery was deemed appropriate if cefazolin was given within 30 minutes before incision, and vancomycin at least 1 hour before incision.3
Secondary Objective
SSI was defined as infection of the skin, subcutaneous tissue, and deep soft tissues (e.g., fascia or muscle) of the incision. It includes one of the following: purulent drainage; organisms isolated from superficial incision cultures; at least one sign of inflammation, for example pain, tenderness, induration, erythema, local warmth of wound; or if a surgeon declared the wound infected.12
The incidence of SSIs within 90 days of surgery and the causative pathogens (isolated from sternal wound/tissue cultures) were compared between groups to evaluate the efficacy of perioperative prophylaxis (comparing 48 hours with 24 hours). All-cause mortality within 30 days post-surgery and post-surgical length of hospital stay were also compared as additional safety indicators.
Statistical Analysis
All statistical analyses performed were two-tailed tests at 5% significance level, using IBM® SPSS® Statistics for Windows Version 25.0 (Armonk, New York, USA). Chi-square or Fisher’s exact tests were used for categorical data. For continuous data, one-way Analysis of Variance was used for normally distributed data, while the Kruskal–Wallis test was used for non-normally distributed data. For post-hoc comparisons, significance level was adjusted via Bonferroni correction. All post-hoc comparisons involved three pairs of comparisons. Hence, significance level was adjusted to 0.0167.
RESULTS
A total of 2,036 patients undergoing cardiothoracic surgery with sternotomy were extracted from the patient database. These procedures were mainly coronary artery bypass surgeries with or without valve surgery. After selecting for every fourth patient, 509 patients were included in the study (Group 1: 149 patients; Group 2: 184 patients; Group 3: 176 patients). Patient demographics were similar across all three groups, and are as presented in Table 2. Most patients were males (87.2%), with a mean age of 62.8±8.6 years.
In general, the surgeons consistently selected the right antibiotics for surgical prophylaxis (>90% across all three groups [ Table 2 ]). A small group of patients received inappropriate choice of antibiotic prophylaxis (e.g., vancomycin in the absence of β-lactam allergy [n=14]), single antibiotic therapy instead of dual vancomycin and cefazolin in MRSA colonised patients post-guideline implementation (n=5), dual antibiotics for prophylaxis in non-MRSA colonised patients out of guideline recommendations (n=10), or receipt of antibiotic prophylaxis other than cefazolin and/or vancomycin (n=2). None of the MRSA-colonised patients in Group 1 received dual antibiotic prophylaxis with vancomycin and cefazolin. Following implementation of revised SAP guidelines, one out of three (33.3%) and two out of seven (28.6%) received dual cover for prophylaxis in Groups 2 and 3, respectively.
Antibiotic prophylaxis was administered in a timely fashion for >85% of the patients in all three groups (Table 2). After reaching out to the cardiothoracic and anaesthesiology teams to communicate the changes in SAP, and to reinforce good practice, the authors observed a trend showing improvement in the proportion of patients who had timely administration of SAP, from 90.6% (Group 1) to 96.7% (Group 2) (p=0.019, not statistically significant after Bonferroni correction). However, this effect had worn off a year later. The proportion of patients who received SAP on time decreased significantly from 96.7% in Group 2 to 87.5% in Group 3 (p<0.001).
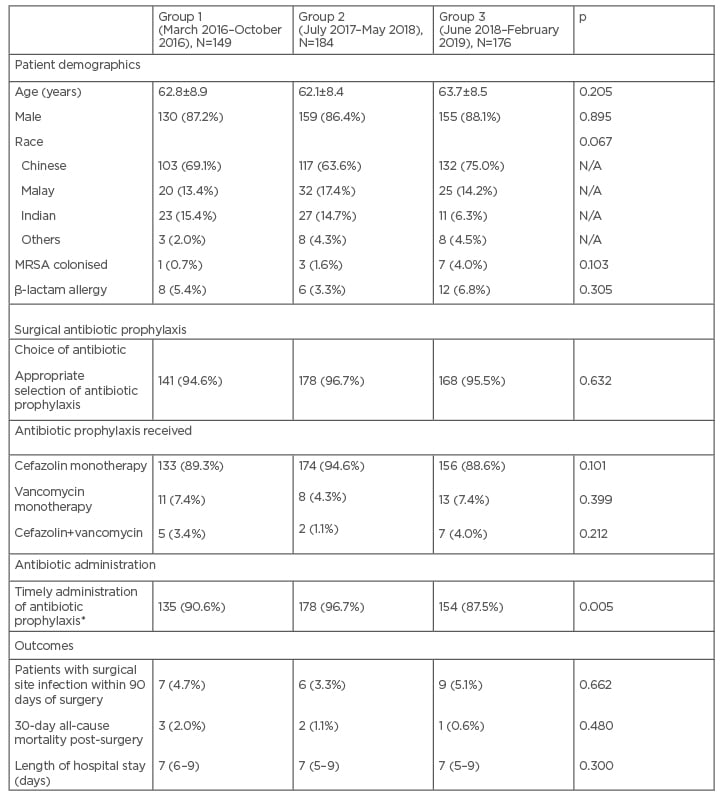
Table 2: Patient demographics, compliance to antibiotic prophylaxis guidelines, and incidence of surgical site infections.
*Cefazolin to be given within 30 minutes of incision; vancomycin to be given at least 1 hour before incision.
Duration of 48 hours was considered compliant based on the previous antibiotic prophylaxis guideline.
The data is presented as mean±standard deviation, or median (interquartile range), or number (percentage), where appropriate.
MRSA: methicillin-resistant Staphylococcus aureus.
After the revised SAP guidelines were implemented, the proportion of patients on prolonged antibiotic prophylaxis (>24 hours) decreased significantly from 149/149 (100.0%) in Group 1 to 55/184 (29.9%) patients in Group 2 (p<0.001). With time, there was a trend towards reverting to old habits of prolonging SAP; the proportion of patients with SAP >24 hours increased from 55/184 (29.9%) in Group 2 to 67/176 (38.0%) in Group 3 (p=0.08) (Figure 1A).
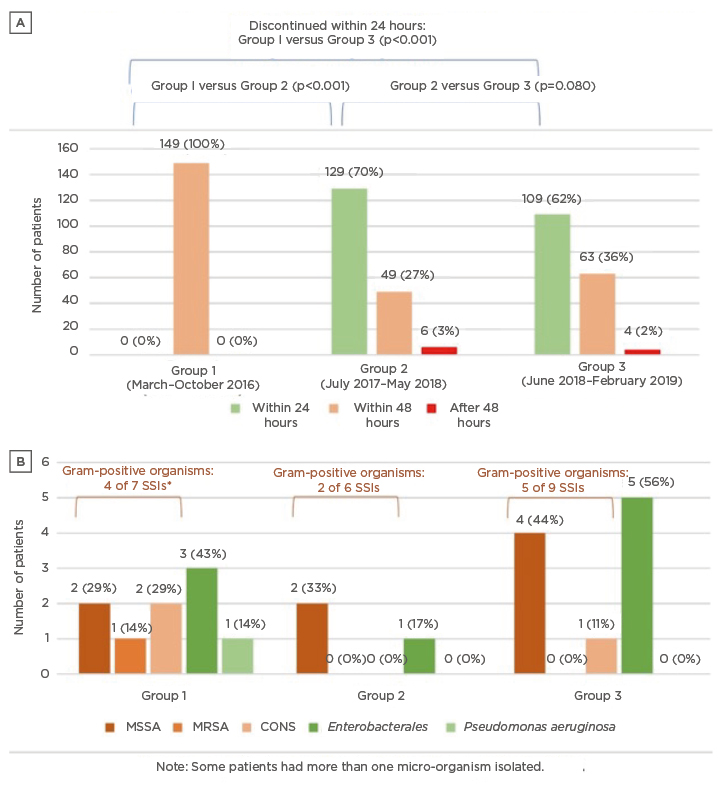
Figure 1: Trends in the duration of surgical prophylaxis duration and surgical site infections before and after guideline implementation.
A) shows the distribution of patients who received 24 hours, 48 hours and >48 hours of surgical prophylaxis across the three groups. After guideline implementation, most patients received surgical prophylaxis for 24 hours instead of 48 hours. B) shows the pathogens from the SSI across the three groups.
*One patient had both CONS and MRSA isolated from their surgical wound site.
CONS: coagulase-negative Staphylococcus; MSSA: methicillin-susceptible Staphylococcus aureus; MRSA: methicillin-resistant Staphylococcus aureus; SSI: surgical site infection.
Despite the reduction in duration of antibiotic prophylaxis from 48 hours to 24 hours since November 2016, the incidence of SSIs remained stable across the three groups (4.7% versus 3.3% versus 5.1%; p=0.662). Similarly, in a separate subgroup analysis of all patients post-guideline implementation (i.e., Groups 2 and 3 combined), there was no difference in the SSI rates among those receiving SAP ≤24 hours versus >24 hours. SSI incidence were 9 (3.8%) versus 6 (4.9%), respectively (p=0.627). After guideline implementation, the authors also did not observe any adverse impact on post-surgical mortality and length of stay (Table 2).
Almost all patients with SSIs received appropriate antibiotics based on guideline recommendations, except for one patient who received dual antibiotic prophylaxis for an individual who is not colonised with MRSA. Of those who developed SSIs, three out of 22 patients (13.6%) did not receive SAP within the correct period; two out of these three cases occurred in patients from Group 1, before the revision and implementation of the SAP in November 2016. These two patients developed coagulase-negative Staphylococcus and MRSA SSIs; vancomycin was not served on time for both cases.
For patients with culture proven SSIs, a significant proportion are caused by Gram-positive organisms such as S. aureus and coagulase-negative Staphylococci as illustrated by Figure 1B. MSSA remains a common causative pathogen for sternal wound infections. Interestingly, MRSA was only isolated before the implementation of the revised SAP guidelines (Group 1), but not in Groups 2 and 3. The authors also did not observe a major shift in susceptibility of pathogens after the implementation of revised SAP guidelines.
DISCUSSION
Whilst it is established that SAP is important for the prevention of SSIs,3 antibiotic misuse for the purpose of perioperative prophylaxis is not uncommon. Although local and international guidelines are available, compliance to SAP guidelines is often variable and suboptimal.13 In clinical practice, SAP is also frequently extended beyond 24 hours, especially in cardiothoracic surgeries.14,15 In this small retrospective before–after single centre study, the authors’ team evaluated the impact of reducing SAP from 48 hours to 24 hours in cardiothoracic surgery. Two things stood out. Firstly, and unexpectedly, they observed a relatively high compliance to a revised SAP guidelines co-developed together with the cardiothoracic surgeons, especially when it was first implemented. Secondly, reduction of SAP from 48 hours to 24 hours did not result in an increase in SSIs.
To ensure that the revised SAP guidelines will be adopted, the antimicrobial stewardship team implemented a multi-prong approach to increase awareness of the updated guidelines, and to optimise the prescription of antibiotic prophylaxis for cardiothoracic surgeries. This involved the direct engagement of cardiothoracic surgeons during review of the SAP, and education roadshows to the departments of cardiothoracic surgery and anaesthesiology to communicate the rationale for changes in guidelines, and inform the team of the changes implemented, including the creation of antibiotic prophylaxis order sets in the electronic prescribing system. In addition, the authors had the head of the cardiothoracic unit working alongside their team, championing this initiative. With this bundled approach, they observed high compliance rates to the updated guidelines in terms of antibiotic choice and duration, immediately after the implementation of our new guidelines. This illustrates the point that evidence alone is unlikely to change practice.16,17 For practice change, it is also necessary to adopt a more collaborative and inclusive approach, engaging stakeholders in the decision making process;16,18,19 address the surgeon’s prescribing bias;16,20 and incorporate electronic tools such as clinical decision support systems to improve prescribing.21 For the cardiothoracic team, apart from evidence-based practice and local guidelines, a surgeon champion was instrumental to ensure that the unit’s concerns were addressed, and goals were aligned.20
Having said this, compliance rates to SAP decreased 18 months after guideline implementation. The initial high SAP compliance rate is probably due to a visible stewardship presence during the initial launch of the revised guidelines. After the revised SAP was implemented, audits were not conducted for antibiotic prophylaxis, and education on appropriate use of antibiotics for prophylaxis was not reinforced thereafter. This phenomenon is not unexpected, and when stewardship presence is withdrawn, antibiotic use or misuse of antibiotics may increase.22,23 Although time-consuming, continued stewardship engagement and regular educational sessions with the surgical teams are crucial.24 In addition, targeted reviews of prescriptions for SAP and feedback may be important for sustained improvements.25
The optimal duration of SAP in cardiothoracic surgeries is not so well established. In a randomised controlled trial, Gupta et al.26 showed that 48 hours of SAP is as effective as 72 hours. In subsequent meta-analyses, Mertz et al.10 and Lador et al.27 reported that SAP for >24 hours reduced the risk of sternal SSIs; however, the studies included for those reviews were heterogeneous and confounded by biases. There is emerging evidence to support a shorter course of prophylaxis (e.g., <48 hours).28,29 Similar to the findings by Surat et al.,29 this study showed that SAP for 24 hours is safe and did not affect the incidence of SSIs. The authors’ SSI rates (3.3–5.1%) were also comparable to these studies (Hamouda et al.28 reported 5.4%, while Surat et al.29 reported 3.6%). There are also other reports supporting shorter courses of SAP to reduce antimicrobial usage and Clostridioides difficile infection.30
By and large, SSIs post-sternotomies are caused by Gram-positive organisms, S. aureus, and coagulase negative Staphylococcus being more common. Based on in-house data, the authors also observed breakthrough infections with MSSA in patients on vancomycin monotherapy for SAP, likely due to poorer activity of vancomycin monotherapy (relative to β-lactam antibiotics) against MSSA.5,9,31,32 This is also reported in the literature. In a USA-based quasi-experimental pragmatic prospective study evaluating SSIs in patients undergoing cardiac, hip, or knee surgery, the rate of complex S. aureus SSIs was in MRSA-colonised patients receiving vancomycin and cefazolin or cefuroxime for perioperative prophylaxis.8 This prompted the guideline revision at the authors’ centre to recommend dual cover, with both vancomycin and cefazolin for patients colonised with MRSA undergoing sternotomies. After this change in practice, they did not have breakthrough MSSA infections in patients colonised with MRSA. The authors acknowledge that this is a small study, and larger studies would be warranted to corroborate observations.
MSSA remained the predominant pathogen in this study, even after the revision of guidelines. In Groups 2 and 3, five out of six MSSA SSIs occurred after prophylaxis with cefazolin monotherapy, while one MSSA SSI occurred after prophylaxis with vancomycin monotherapy. This suggests that appropriate antibiotic prophylaxis is not the only solution in preventing SSI, as the aetiology of SSI can be multi-factorial.33 Additional interventions beyond the scope of antibiotic prophylaxis, may need to be evaluated and considered to further reduce SSI rates.33
Appropriate timing of administration for perioperative prophylaxis also plays a role in reducing incidence of SSIs. In this study, three out of 22 patients with SSIs (13.6%) did not receive perioperative antibiotics within the correct timeframe. With incorrect timing, there may be ineffective plasma and tissue antibiotic concentrations, increasing risk of SSIs as proven by Zelenitsky et al.34 While compliance to this aspect of the guidelines improved significantly immediately after guideline implementation, there was a significant decline in compliance in Group 3. This highlights the need for regular reminders and continued engagement with the surgical teams for continued compliance.
LIMITATIONS
While the authors had positive findings demonstrating high surgical compliance to SAP and stable SSI rates with 24 hours of SAP, they acknowledge that this is a small retrospective study with potential for recorder bias. Given the small sample size and low incidence of SSIs, they cannot comment on shifts in the epidemiology of SSIs during the study period. Also, the complexity of the cardiothoracic surgeries was not graded in this study, and this could be one of the confounders affecting SSI rates.
CONCLUSION
A bundled approach to SAP in cardiothoracic surgery (guideline update, provider engagement or education, and creation of electronic order sets) was effective at this centre in improving compliance to SAP. While there was a significant reduction of SAP from 48 hours to 24 hours, there was a creep in proportion of patients with extended SAP (>24 hours) with time, highlighting the importance of continued engagement with cardiothoracic surgeons by the stewardship team. The authors’ data has shown that reduction of surgical prophylaxis to 24 hours is effective and safe, without any increase in incidence of SSIs.







