Abstract
Chronic total occlusions (CTOs) are detected incidentally in ˜20% of patients undergoing coronary angiography and are often associated with significant morbidity and mortality. CTOs can manifest with worsening symptoms, reduced left ventricular function, and increased incidence of ventricular arrhythmias. Despite this, according to USA, Italian, and Japanese national registry data, only ~5–22% of CTO lesions are treated by percutaneous coronary intervention (PCI). CTO-PCI is a particularly challenging technique for this subset of lesions and has traditionally been associated with increased risks and complications compared to conventional PCI. However, increased experience, the development of novel techniques, and dedicated equipment have revolutionised CTO-PCI. USA, Italian, and Japanese registry data have shown success rates of between 85% and 90%, with diminishing complication rates when performed by experienced operators. Moreover, observational studies have suggested that there are significant benefits of using CTO-PCI, including fewer symptoms, improved quality of life, reduced need for coronary artery bypass surgery, and reduction in ischaemic burden and mortality. In addition, when there is demonstrable ischaemia and viable myocardium in the CTO territory, there is further potential prognostic benefit from complete revascularisation. However, there has so far been a relative lack of randomised trial data to support the routine use of CTO-PCI. This paper reviews the current evidence surrounding this subject and discusses the arguments for and against CTO-PCI. It includes an exploration of the interventionalist’s ‘toolbox’ and the techniques used in CTO-PCI, including a section on ‘tips and tricks’ for the most challenging cases. Finally, there is a discussion on the future of CTO-PCI including promising ongoing clinical trials and novel equipment that may improve outcomes and help to establish a more widespread adoption of CTO-PCI.
BACKGROUND AND HISTORICAL PERSPECTIVE
“If I had an enemy I would teach him angioplasty,”1 were the words uttered by Andreas Gruentzig in 1980, 3 years after he performed the first percutaneous coronary intervention (PCI) in September 1977. Andreas Gruentzig would have witnessed the clinical benefits subside over time and, as the reality dawned that adverse events do occur post PCI, the initial honeymoon period and optimism of using PCI would have been diminished. Through further technological development, operator skill, and pharmacotherapy, PCI has now become the mainstream of treatment and the default choice for most revascularisation procedures. However, the last frontier of PCI that fills most operators with trepidation is chronic total occlusion (CTO)-PCI. There has been a large increase of innovations that have allowed the interventional cardiologist to treat diseases that were once only amenable to surgery.
CTOs are a subset of coronary artery disease (CAD) defined as coronary arteries with absent anterograde flow over >12 weeks duration.2 They have been found in ≤20% of coronary disease cases diagnosed at angiography.3 The technical difficulty associated with treatment of CTOs has led to the conclusion that it is the ‘final frontier’ in interventional cardiology.3-5 The low intervention rates are commonly due to the misconception that intervening on a CTO will have limited benefit, with higher complication rates. CTO-PCI is no enemy, but a friend, and we present here the evidence, treatment strategies, and indications that allied to newer devices and techniques, ensure safe and effective treatment of these complex lesions, and often forgotten patients.
JUSTIFICATION
Werner et al.6 demonstrated that regardless of the degree of collateralisation, the area of myocardium distal to the CTO lesion is always ischaemic. Ischaemia is effectively relieved by a successful CTO-PCI, although success depends on the vessel intervened upon.7,8 Additionally, complete, as opposed to incomplete, revascularisation has mortality benefits regardless of whether surgical or percutaneous revascularisation is used, supporting the notion that CTO-PCI reduces ischaemic myocardial burden (Figure 1).2
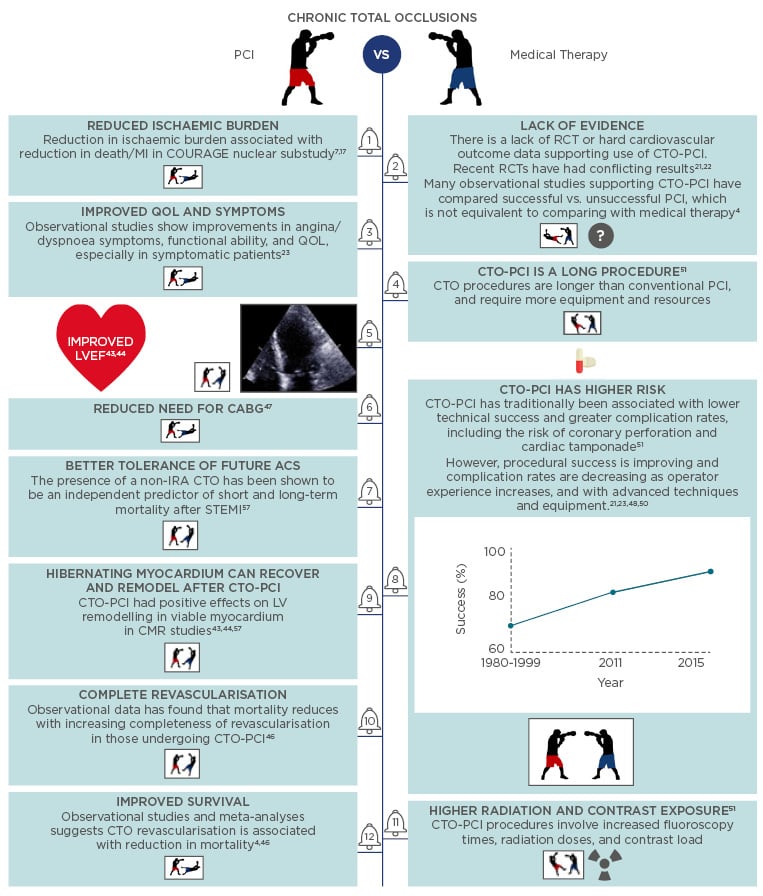
Figure 1: The boxers’ guide to CTO-PCI: A selection of arguments for and against CTO-PCI.4,8,44-54
PCI: percutaneous coronary occlusion; MI: myocardial infarction; IRA: infarct related artery; CTO: chronic total occlusion; CABG: coronary artery bypass grafting; ACS: acute coronary syndrome; RCT: randomised controlled trial; LVEF: left ventricular ejection fraction; QOL: quality of life.
Intervention has been linked to a lower incidence of ventricular arrhythmias,9 improved left ventricular (LV) function,10 and improvements in quality of life (QoL).11 A 2017 study by Sotomi et al.12 examined the effects of CTO-PCI on electrical stability and LV function in a single-centre, prospective, observational study. It evaluated electrical stability as a surrogate marker for ventricular arrhythmias, using signal-averaged electrocardiogram (SAECG), and assessed systolic and diastolic function using speckle tracking echocardiography. They found no improvements in electrical stability following CTO-PCI, but did find some evidence of enhancements in systolic and diastolic function.
ISCHAEMIA
Clinically, the primary driver towards CTO-PCI is symptomatic angina with associated coronary ischaemia. The American cardiovascular societies’ appropriate use criteria (AUC) outlines 180 clinical scenarios aiming to guide clinicians in making decisions about intervention in patients with CAD including CTOs.13 The AUC heavily emphasise ischaemic burden as a reason for revascularisation when there is a large area of ischaemia, even in the absence of symptoms. The 2014 European revascularisation guidelines recommend that CTO-PCI be considered when there is an expected reduction in myocardial ischaemia (Class IIa, Level of Evidence B).14,15 CTO-PCI has been shown to significantly decrease the ischaemic burden from 13.1% to 6.9%. Moreover, mortality has been shown to increase in several studies if the percentage of myocardium at risk is >7–10%.16,17
SYMPTOMS
A key objective of CTO-PCI is to induce relief of symptoms, improve exercise capacity, and improve QoL. Anecdotally there is a tendency to underestimate the symptoms attributable to CTOs. This is because there tends to be a higher prevalence of angina chest pain rather than shortness of breath.2 Large observational studies looking at the impact of CTO revascularisation have found improvements in patient’s angina and QoL (assessed by QoL indexes).18-20 The beneficial effects include improved physical activity (p=0.01), rarer anginal episodes (p<0.001), and greater treatment satisfaction (p=0.03) compared with failed interventions.20
Considering the importance of symptom control on the benefit of CTO intervention, few studies exist that compare its efficacy with optimal medical treatment (OMT). DECISION CTO21 is a randomised controlled trial (RCT) which began recruitment in 2010 and presented its findings at the American College of Cardiology (ACC) scientific sessions in March 2017. It recruited 834 patients and was due to follow-up for a total of 5 years; however, due to problems with recruitment it stopped at 3 years. The CTO-PCI success rate was 91.1%. At 3 years, the combined primary endpoint of all-cause death, myocardial infarction (MI), stroke, and repeat revascularisation in the intention-to-treat population was similar for patients on OMT and CTO-PCI (19.6% versus 20.6%; p=0.008 for non-inferiority). Furthermore, there were no differences in angina or QoL scores at 1-year.
This study contrasts that of the EUROCTO22 club trial which began recruiting in 2012 and presented its 12-month findings in May 2017 at the EuroPCR congress. It was a multicentre RCT, which examined the symptomatic benefit of CTO-PCI versus OMT (aspirin, statin, angiotensin-converting-enzyme [ACE] inhibitor, and two anti-anginals at the maximum tolerated dose). The study recruited 407 patients from 26 different countries. Using the Seattle Angina Questionnaire (SAQ) it showed a benefit of using CTO-PCI versus OMT (p=0.009) and reported improvements in the Canadian Cardiovascular Society (CCS) angina score (p<0.001). It also showed that improvements in QoL, angina stability, physical activity, and treatment satisfaction were numerically higher in CTO-PCI compared with OMT. There were comparable MACCE rates at 12 months.23
DECISION-CTO and EUROCTO highlighted the continued controversy about trial data which has led to a divergence in the real-world management of CTOs across different institutions and countries.
CLASSIFICATION AND SCORING SYSTEMS
Scoring systems help to predict the probability of procedural success. The 2011 Japanese CTO (J-CTO) is based on a multicentre retrospective analysis of nearly 500 CTO procedures. It identified five independent predictors of the ability of the guidewire to cross the lesion within the first 30 minutes.24 It applies one point for each of these variables when present: i) calcification; ii) bending >45° in the CTO segment; iii) a blunt proximal cap; iv) when the length of the occluded segment is >20 mm; and v) a previously failed attempt. The CTO case complexity was further stratified into easy (J-CTO score=0), intermediate (J-CTO score=1), difficult (J-CTO score=2), and very difficult (J-CTO score=3–5) (Figure 2).
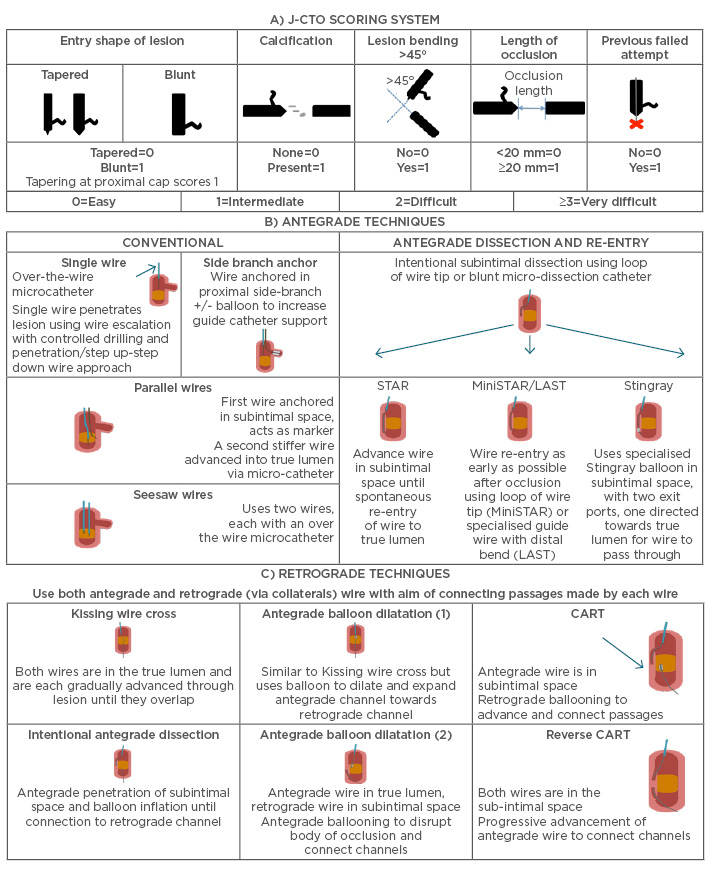
Figure 2: A) J-CTO score is calculated by addition of individual scores, allowing lesions to be categorised as easy, intermediate, difficult, or very difficult; B) and C) Antegrade and retrograde techniques in CTO-PCI.52,53
CTO: chronic total occlusion; PCI: percuateous coronary intervention; CART: controlled antegrade and retrograde tracking.
Adapted from Morino et al.24
The J-CTO study had limitations, including the fact that the overall success rate was 72% versus the current standard of 85–90%,24,25 and the under-representation of retrograde PCIs. Alessandrino et al.26 proposed the Clinical and Lesion (CL) related score. This emerged from a single-centre prospective trial of 1,657 patients. It includes variables such as the anatomy of the proximal cap, grade of lesion calcification, left anterior descending (LAD) or non-LAD CTO, lesion length >20 mm, and history of coronary artery bypass grafting or MI. All variables are then adjusted on the basis of an odds ratio.
ORA,27 PROGRESS-CTO,28 and RECHARGE29 are more contemporary scoring systems, taking into account the hybrid approach. The ORA scoring system uses ostial location, Rentrop Grade <2 (Grade 0 has no angiographic evidence of filling and 4 is fully filled), and age ≥75 years; with these measurements it is possible to assign each lesion a technical difficulty and therefore predict procedural success.27 The PROGRESS-CTO score also uses four variables (proximal cap ambiguity, absence of retrograde collaterals, moderate or severe tortuosity, or a circumflex CTO). This was developed from the PROGRESS registry and was validated against the J-CTO scoring system. The RECHARGE29 score uses six variables (blunt stump, calcification, tortuosity >45°, lesion length >20 mm, diseased distal landing zone, and previous CTO vessel bypass graft) and was validated against the PROGRESS and J-CTO scoring systems.
Yu et al.30 have developed the Korean Multicentre CTO CT Registry (KCCT) score in 2017. Unlike the aforementioned scores, it uses computed tomography (CT) angiographic imaging to predict the difficulty of navigating the CTO within 30 minutes as well as procedural success. It analysed 684 CTO lesions with CT and identified proximal blunt entry, proximal side branch, bending, occlusion length >15 mm, severe calcification, whole luminal calcification, reattempt and >12 months or unknown duration of CTO as being independent predictors of success. A KCCT score <4 predicts the ability to cross the lesion within 30 minutes (p<0.05).
‘THE KIT’
Vascular Access
The femoral approach allows larger and more supportive guiding catheters; however, radial access increases patient comfort and reduces the incidence of vascular complications. With complex lesions (J-CTO >3) we recommend long 8 Fr and 7 Fr femoral sheaths. The Arrow® long armoured-style sheath is particularly useful for this and also has great trackability in patients with peripheral vascular disease. This will ensure a hybrid approach is feasible, if necessary, and will also allow the insertion of intravascular ultrasound (IVUS), microcatheters, guideliners, anchor balloons, and multiple wires and balloons if required.
Guide Catheters
Guide catheter selection is extremely important; if the procedure requires additional support to prevent prolapse then a larger diameter catheter should be used (AL1 for the right coronary artery, and EBU for the left coronary artery, usually with preference to size up [e.g. use EBU4 in preference to EBU3.5]). Softer tip catheters are emerging such as Hyperion™ (Asahi Intecc, Aichi, Japan), which may cause less trauma to the ostia and are marginally more steerable. In general, two guide catheters are used for each CTO-PCI case to allow the operator to seamlessly switch between antegrade and retrograde approaches. A mandatory dual coronary injection is performed to assess: i) a clear understanding of the location of the proximal cap; ii) occlusion length; iii) the presence of side branches and size and quality of the distal vessel; and iv) the presence of collaterals suitable for retrograde technique.
Wires and Microcatheters
Failed CTO-PCI is frequently caused by the inability to cross the CTO with a guidewire. There are three separate steps required to cross a CTO: i) penetrating the proximal fibrous cap; ii) traversing the body of the CTO to reach the distal fibrous cap; and iii) penetrating the distal fibrous cap. Dedicated guidewires for crossing CTOs have been developed. They are broadly divided into two groups:
Hydrophilic (polymer coated) wires (e.g. Fielder™, Fielder XT [Asahi], Pilot 200® [Abbott, Illinois, USA]) – offer manoeuvrability in tortuous vessels and passage through micro-channels into the true lumen. They can, however, increase the incidence of sub-intimal perforation.
Non-hydrophilic (non-polymer coated) wires (e.g. Miracle Bros® 3-12 [Asahi], Confianza Pro [Asahi]), are typically more controllable, provide better tactile feel, and are less likely to cause vessel dissection. They can be used to cross the fibrous cap as they have greater penetration force. They are graded in grams according to the penetration force they can withstand in grams during bench testing. This does give an idea of penetrability ex vivo, but operators should be cautious that, in regard to in vivo with microcatheter support, these values are only a guide and can be higher if used with anchor balloons or differing microcatheters. There are also newer steerable intra-occlusion wires such as the Gaia family (Asahi) (first, second, and third). The unique property of these wires is their ability to steer the wire with the use of a microcatheter within the occlusion.
It is possible to cross the CTO with a guidewire alone, although in the majority of J-CTO >2 cases a microcatheter for support is considered mandatory (e.g. Corsair [Asahi], FineCross® [Terumo, Leuven, Belgium], M-CATH [Accrostak, Geneva, Switzerland], NHancer [IMDS, Roden, Netherlands], Turnpike [Vascular Solutions, Minnesota, USA]). This allows the operator to provide increased wire force to the proximal fibrous cap. An alternative is an over-the-wire (OTW) balloon catheter (e.g. 1.25–1.5 balloon diameter), which, allied to increase force, allows pre-dilation once the CTO is crossed. OTW has more or less disappeared from use in CTO-PCI work and is essentially historic because the main limitation is the inability of OTW to track through complex CTOs.
‘TIPS AND TRICKS’ AND CHRONIC TOTAL OCCLUSION TECHNIQUES
The Stingray Balloon and Wire
When unable to penetrate the fibrous cap, a dissection flap can be created to circumnavigate the occlusion. The Stingray™ LP balloon (Boston Scientific, Massachusetts, USA) is a flat balloon that can be inflated in an intended dissection plane. There are two radiopaque markers on the Stingray balloon and an exit hole just proximal to each marker on the opposite face of the balloon. One exit port will lead the wire into the adventitial side of the artery distal to the occlusion and the other to the luminal side, therefore allowing re-entry and bypass of the CTO. The Stingray Wire (Boston) is a stiff wire with a pre-shaped 28° tip and a barb on the end to facilitate re-entry. It can be carefully advanced in the Stingray balloon and directed towards either the adventitia or luminal layer.
Anterograde
Anterograde is the most common approach to CTO-PCI. Progressively stiffer hydrophilic/non-hydrophilic wires are used sequentially until the proximal cap is penetrated or the wire advanced within the lesion. Failure after initial wire entry in the lesion has led to specialised techniques for recanalisation of CTOs. The antegrade dissection/re-entry technique (ADR) and subintimal tracking and re-entry (STAR) techniques (Figure 2) were pioneered by the work of Colombo et al.31 and Carlino et al.32 The STAR technique is a less well-controlled method of performing an ADR, where a hydrophilic wire in the shape of an ‘umbrella handle’ is knuckled though the intimal dissection planes until it enters the distal true lumen. ADR with the use of the aforementioned Stingray balloon and Crossboss™ (Boston) device allow the operator to perform a more precise and limited dissection than what was previously observed with the STAR technique. These techniques have been essential in the remarkable improvements in the procedural success rates.
Retrograde
A retrograde approach can be broadly described as any approach that uses donor collaterals to deliver interventional equipment to facilitate the opening of a CTO. Unlike early procedures, this procedure has evolved to use septals (which have a straighter course) or the more tortuous epicardial vessels as the common conduit arteries.33 The goal of the retrograde approach is to target the distal cap, which could be softer than the proximal one. It should be noted that the retrograde approach should only be performed by experienced operators who have mastered the anterograde approach. For all CTO-PCI, the activated clotting time (ACT) should be measured throughout ensuring an ACT >300, and further heparin introduced if required.
Various techniques have been described for retrograde CTO-PCI. These include passing the guidewire retrogradely through the distal cap within the true lumen with balloon dilatation before antegrade guidewire passage and PCI. Alternatively, passing the wire into the subintimal space and enlargement with sequential balloon dilatations before connecting with the true lumen retrogradely (controlled antegrade and retrograde tracking [CART] technique). If the subintimal space is enlarged from the anterograde then it is called the reverse CART technique (Figure 2). If the retrograde wire and microcatheter is eventually inserted into the antegrade guide then the RG3® (Asahi) wire is utilised to pass through the occlusion and is externalised in the opposite sheath. Conventional PCI can then be performed on the RG3 wire in an antegrade manner. It must be emphasised that at no time should contrast injections be performed in the antegrade guide prior to stenting, as this will only facilitate dissection planes and can cause large haematomas that could lead to compression of cavity and haemodynamic compromise.
The Hybrid Approach
The hybrid approach was proposed in 2012 and has been shown to be effective in ≤90% of cases with fewer complications (cardiac tamponade: 0.4%, periprocedural MI: 1.0%, death: 0.4%).9,34 This is effectively a combination of the anterograde and retrograde techniques described above.
Future Directions in Chronic Total Occlusion-Percutaneous Coronary Intervention
Two further ongoing clinical trials should further clarify the benefits of CTO-PCI. OPEN-CTO35 is a prospective observational registry of patients enrolled in North America utilising the hybrid approach. It aims to assess the safety, health and QoL, and cost effectiveness of the method. Initial results have been promising (Table 1).
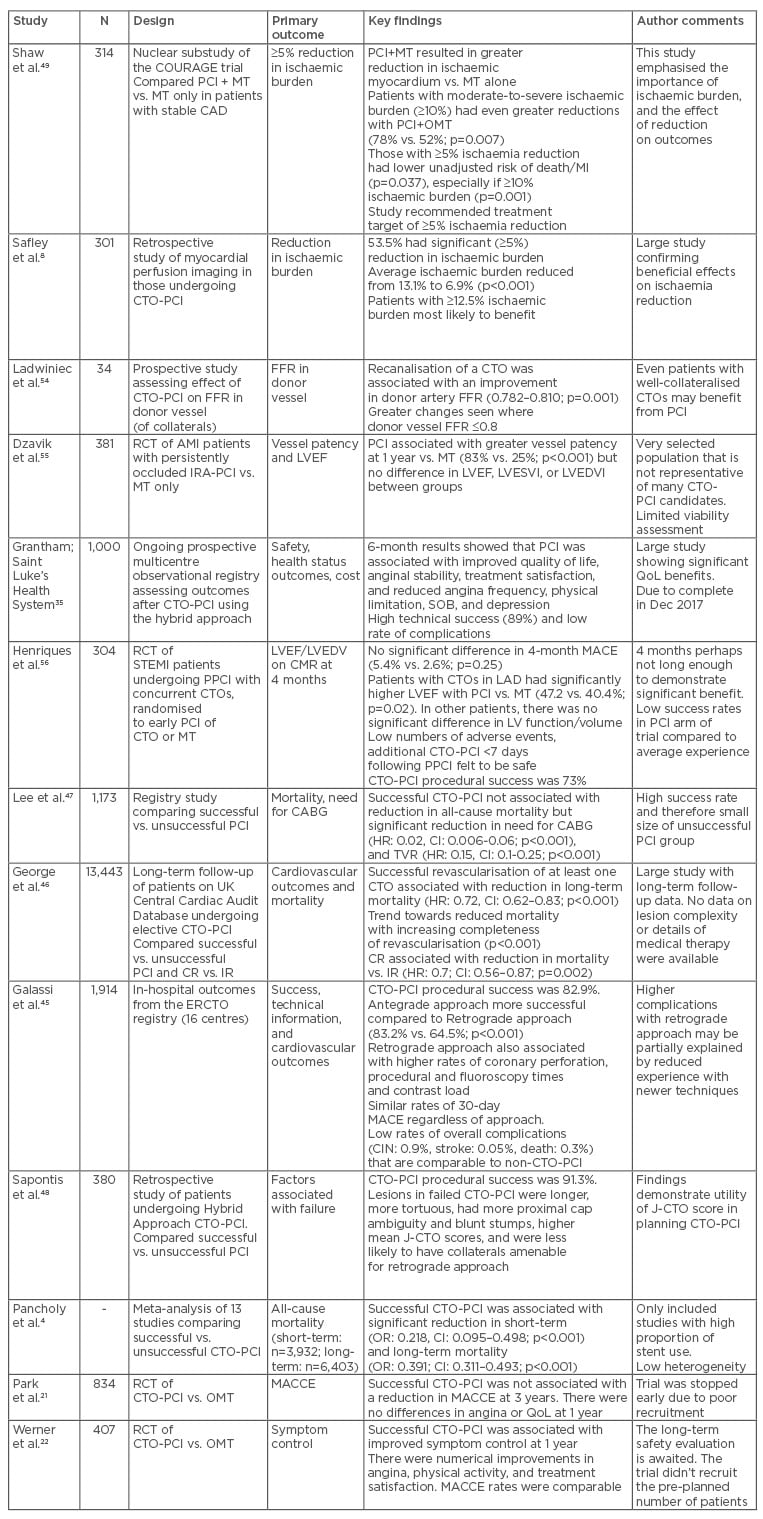
Table 1: A summary of a selection of important studies relating to CTO-PCI.
AMI: acute myocardial infarction; CAD: coronary artery disease; CI: confidence interval; CIN: contrast-induced nephropathy; CR: complete revascularisation, CTO: chronic total occlusion; ERCTO: European Registry of Chronic Total Occlusion; FFR: fractional flow reserve; IR: incomplete revascularisation; LVEDV: left ventricular end diastolic volume; LVEDVI: left ventricular end-diastolic volume index; LVEF: left ventricular ejection fraction; LVESVI: left ventricular end-systolic volume index; MACE: major adverse cardiovascular events; MT: medical therapy; n: number of patients; PCI: percutaneous coronary intervention; QoL: quality of life; RCT: randomised controlled trial; TVR: target vessel revascularisation; OMT: optimal medical therapy; MI: myocardial infarction; J-CTO: Japanese CTO; LAD: left anterior descending; HR: hazard ratio; OR: odds ratio; MACCE: major adverse cardiovascular and cerebrovascular event.
SHINE-CTO36 is a USA single-centre RCT of CTOs to PCI versus sham procedure, and will assess the impact on QoL.
TECHNOLOGICAL DEVELOPMENTS AND THE SPECIFICS TO CHRONIC TOTAL OCCLUSION-PERCUTANEOUS CORONARY INTERVENTION
Technological developments may provide the ‘knockout blow’ for CTO-PCI. Some promising developments are described below.
Orbital Arthrectomy
Orbital atherectomy can facilitate plaque removal and softening, improve lesion compliance, and aid dilatation, especially in severely calcified lesions.37 Though underused, it has been shown to be effective at facilitating angioplasty in resistant CTOs where device crossing is initially unsuccessful.38
Drug-eluting Balloons
Drug-eluting balloons have been the target of research looking at lowering the rates of in-stent restenosis. They also require a shorter duration of antiplatelet therapy. Koln et al.39 investigated the feasibility and safety of its use in CTOs. They looked at 66 cases of anterograde CTO intervention (retrograde was not investigated due to the high prevalence of dissection). They found that restenosis (11.8%) and reocclusion (5.9%) rates were comparable to CTO-PCI. This was a small, non-randomised study and the lesions themselves had to have had good predilation results, which biased their selection. In view of its potential, further RCT evidence is required.
SoundBite
The SoundBite Crossing system40 (SoundBite Medical Solutions, Inc, Quebec, Canada) is a novel device using shockwaves (short-duration, high amplitude pressure pulses) to facilitate crossing the proximal cap. It propagates shockwaves to its tip, using it as a micro ‘jackhammer’ to penetrate the lesion, and has recently undergone an ex vivo trial in an amputated leg. Further trials in vivo are expected. Although it is currently being used in peripheral vascular disease, it has potential for use in coronary disease.
Collagen and Collagenase
A collagen-rich matrix is present in CTO lesions and forms a barrier at the proximal cap. Collagenase is an enzyme that degrades Type 1 collagen, and may facilitate lesion crossing. Phase I studies have shown it to be a feasible and safe therapy.41
CONCLUSIONS
CTO’s have a significant impact on health and mortality, yet are undertreated. CTO-PCI offers potential benefits and should be considered for patients with symptoms of, or demonstrated, ischaemia, and viable myocardium in the CTO territory. Modern techniques and scoring sytems allow operators to successfully tackle complex and challenging CTOs. PCI success rates and safety are improving with contemporary trials providing evidence to challenge common misconceptions and allow patients, who may traditionally have been left untreated, to gain the potential benefits from CTO-PCI.








