The Edwards CardiobandTM Mitral Valve Repair System (Edwards Lifesciences Corporation, Irvine, California, USA) enables transcatheter implantation of an adjustable repair device in mitral or tricuspid valve position. The safety and performance for treatment of functional mitral regurgitation have been documented. We now report the initial safety and feasibility results for treatment of functional tricuspid regurgitation (TR).
Twenty consecutive patients with symptomatic TR Grade 3 or 4 were enrolled at five sites in Europe between October 2016 and April 2017. A comprehensive echocardiographic and computed tomography (CT) cardiac screening protocol was used in all patients to assess technical feasibility and to define anatomic landmarks, especially to evaluate the vicinity of the right coronary artery to the tricuspid annulus. All echocardiographic data were analysed by an independent core-lab. To date, 20 patients at high surgical risk (EuroSCORE II: 5% [2–13]) were prospectively analysed. In the cohort, 75% of patients were female, the mean age was 75 years (range: 56–84), and 95% of patients presented in New York Heart Association (NYHA) Class III–IV at baseline. A large proportion of treated patients presented with more-than-severe TR, so-called torrential TR, with vena contracta diameters of >1.5 cm as evaluated by transthoracic echocardiography.
Acute procedural success was defined as successful access, deployment, and positioning of the Edwards Cardioband implant and septo-lateral reduction during the procedure and at discharge, was achieved in all patients. The septo-lateral diameter was reduced by ~27% in all patients after size adjustment of the Edwards Cardioband device. The reduction of septo-lateral diameters remained stable at 30-day follow-up. Echocardiography at 1-month follow-up showed a 44% reduction in Proximal Isovelocity Surface Area effective regurgitant orifice area (PISA EROA) (from 0.9±0.5 cm2 to 0.5±0.3 cm2) and a 29% reduction in vena contracta (from 1.4±0.2 mm to 1.0±0.5 mm).
At 30 days, 73% of patients were in NYHA functional Class ≤2 and quality of life, as assessed by the Kansas City Cardiomyopathy Questionnaire, had significantly improved. Two patients died during the first 30 days after the procedure: the deaths were not directly related to the device or the procedure itself. No further major adverse events were reported for the first 30 days after the procedure.
Although the procedure is challenging for both interventional and imaging cardiologists, it is safe and effective for the reduction of functional TR. Patient selection using multimodality imaging is crucial. Intraprocedural echocardiographic guidance, specifically the use of three-dimensional echocardiography and real-time multiplanar reconstruction views, calculated from three-dimensional volumes of the tricuspid annulus, facilitate the procedure. Of note, the development of a meaningful definition of the most relevant clinical endpoints for TR patients is urgently needed. Furthermore, TR assessment and grading algorithms need refinement.
Early 30-day outcomes suggest a high feasibility and a good safety profile of the Edwards Cardioband Mitral Valve Repair System in selected patients with significant and symptomatic functional TR. Effective acute reduction in TR severity was achieved in all patients and resulted in a significant reduction of septo-lateral diameters in all patients. Further long-term effects of the Edwards Cardioband Mitral Valve Repair System on TR and functional outcomes have to be validated in the ongoing first-in-man trial.
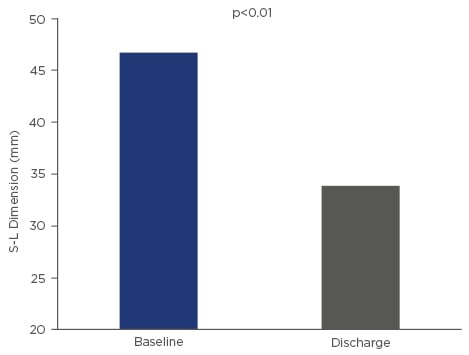
Figure 1: Acute changes in S-L diameter after tricuspid annuloplasty with the Edwards Cardioband device.
S-L: septo-lateral.








