The utilisation of drug-eluting stents (DES) has reached a large diffusion for treatment of coronary artery disease.1 However, patients with diabetes mellitus remain a challenge for in-stent restenosis and adverse cardiovascular events such as late stent thrombosis (ST).2 This is particularly the case after discontinuation of thienopyridine therapy. Despite this, the most recent generation of DES, with the introduction of biodegradable polymers and bioresorbable structure, has reduced the incidence of restenosis in diabetics and cases of ST and is thus confirmed to be the best strategy.3-5 The polymer-free drug-eluting Cre8™ stent (CID, Saluggia, Italy) has three distinctive features: abluminal reservoir technology, bio-inducer surface, and amphilimus formulation (sirolimus and organic acid). The lack of a polymer avoids inflammatory triggers and the drug elution is controlled and directed exclusively towards the vessel wall; the bio-inducer surface is a second-generation pure carbon coating covalently bonded to the thin cobalt-chromium platform, minimising prothrombotic stimuli and promoting a fast stent-strut coverage. The amphilimus formulation (sirolimus and organic acid eluted together) results in a more efficient combined effect, enhanced drug bioavailability, permeability, and maximised product safety and efficacy. This effect is more evident in diabetic cells, due to the overexpression of membrane protein that leads to higher fatty acid binding and translocation.
In the NEXT randomised study, patients treated with the Cre8 stent showed a significantly lower rate of in-stent late lumen loss (LLL) at 6 months compared with Taxus Liberté®, with a trend toward better 12-month clinical safety and efficacy results.6 In the diabetic subgroup the absolute difference in LLL reduction was 72% (0.12±0.29 versus 0.43±0.41). In the PARTICIPATE study the objective was to evaluate the safety and efficacy performances of Cre8 in patients compared with everyday clinical practice, with a specific focus on diabetic individuals.7 The device-oriented major adverse cardiac events rate at 1 year was low and similar in non-diabetic and diabetic groups (coronary intervention target-lesion revascularisation (TLR) 1.0% versus 1.4%) with a LLL of 0.16±0.13. The RESERVOIR trial compared the efficacy of amphilimus-eluting stents (AES) with that of everolimus-eluting stents (EES) in patients with diabetes mellitus.8 The primary endpoint, neointimal (NI) volume obstruction, was 11.97±5.94% for AES versus 16.11±18.18% for EES, meeting the non-inferiority criteria (p=0.0003). By quantitative coronary angiography, in-stent LLL was 0.14±0.24 for AES versus 0.24±0.57 mm for EES (p=0.27), with a larger minimal lumen diameter at follow-up for AES (p=0.02). The multicentric and retrospective registry of polymer-free DES (INVESTIG8 registry) enrolled all consecutive patients who had undergone percutaneous coronary intervention with the Cre8 stent.9 The safety and efficacy of the Cre8 stent were evaluated in an all-comer patient population in terms of incidence of clinical composite endpoint (cardiac death, target-vessel myocardial infarction [MI], and TLR clinically driven) at 12-month follow-up. Secondary endpoints were the incidence of clinical composite endpoint (all deaths, all MI, any revascularisation) and ST (according to an Academic Research Consortium [ARC] definition) at 12-month follow-up. Moreover, a pre-specified analysis in diabetic patients was performed. No inclusion or exclusion criteria were used.
Out of 647 patients from six centres who received the Cre8 stent, 589 patients completed a 1-year follow-up. Forty-five point five percent of patients were found to have acute coronary syndrome and the remainder had stable angina or silent ischaemia. The cardiovascular risk factors were largely diffuse; of particular importance, 21.9% of the diabetic patients (who made up 47.4% of the total patient cohort) required insulin treatment. B2-C coronary lesions were found in 57.8% of patients. In the overall population, at 1-year follow-up, the incidence of the composite endpoint was 4.2% and cardiac death, target-vessel MI, and clinically-driven TLR were 2%, 0.7%, and 1.5%, respectively. The incidence of definite ST was 0.3% (acute ST 0.1% and subacute ST 0.1%) and the probable ST was 0.7% (subacute 0.3% and late ST 0.3%). Kaplan–Meier analysis estimated the rate of survival free from events at 95.7% at 1 year (Figure 1).
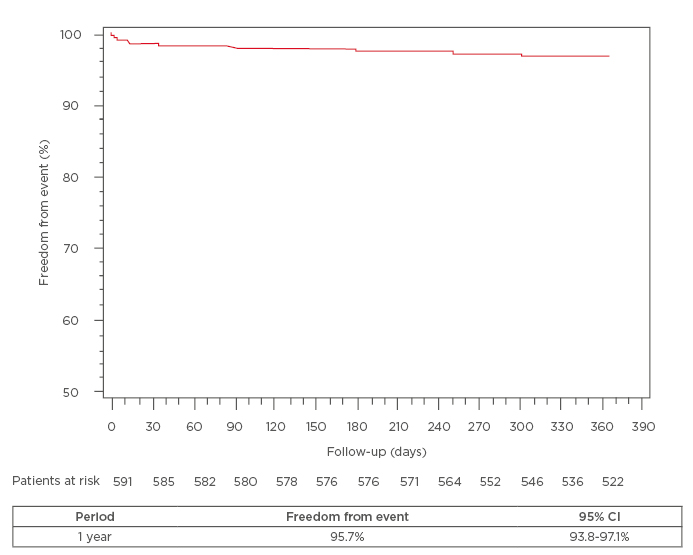
Figure 1: In overall population, Kaplan–Meier analysis estimated the rate of survival free of events
of 95.7% at 1 year.
CI: confidence interval.
The subanalysis in diabetic patients (n=278) showed a more complex clinical risk profile than in non-diabetic patients. At 1-year follow-up, the incidence of composite endpoint was 5% versus 3.5% (p=0.48), cardiac death (2.5% versus 1.6%, p=0.56), target-vessel MI (1.1% versus 0.3%, p=0.34), and clinically driven TLR (1.4% versus 1.6%, p=1), respectively in diabetic and non-diabetic patients. The rates of definite and probable ST did not present a significant difference (definite ST was 0.3% versus 0.3%, p=1; probable ST was 1.1% versus 0.3%, p=0.35). Kaplan–Meier curves estimated a rate of survival free from events of 95.6% versus 97.8%, p=0.15) at 1 year, respectively in diabetic and non-diabetic patients (Figure 2).
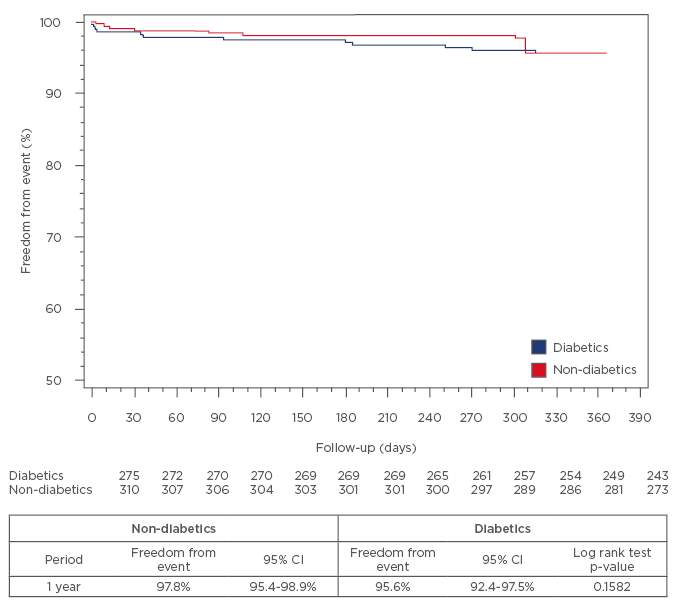
Figure 2: Kaplan–Meier curves estimated the rate of survival free of events of 95.6% versus 97.8%
(p=0.15) at 1 year in diabetic and non-diabetic patients, respectively.
CI: confidence interval.
In conclusion these data demonstrated the low incidence of adverse events confirming the safety and efficacy of Cre8 in a non-selected population at 1-year follow-up. In the diabetic patient subgroup, results showed a good safety profile with the same incidence of clinically driven TLR, even in the presence of a more complex angiographic profile and comparable composite endpoint and ST values.








