BACKGROUND AND AIMS
Bioresorbable vascular scaffolds (BRS) are a new and promising technology. BRS have been developed to provide the same advantages as drug-eluting stents, including radial strength, prevention of vessel recoil, and release of an antiproliferative drug.1 In addition, their complete degradation could offer restoration of vessel vasomotion and endothelial function, reduction of neoatherosclerosis,2,3 and the possibility of future revascularisation with bypass graft if needed. The aim of the present study is to assess the acute and mid-term outcomes of percutaneous coronary intervention (PCI) with magnesium-based BRS in patients with long, diffuse, and complex coronary lesions.
METHODS AND RESULTS
The authors present a 10-case series and included patients presenting with stable coronary artery disease or acute coronary syndrome with complex coronary lesions who needed to undergo PCI. All patients consented to participate in this study.
Exclusion criteria included the presence of distal left main lesions, saphenous vein graft lesions, unsuccessful pre-dilatation, and a reference vessel diameter <2.8 mm or >4.1 mm by quantitative coronary analysis. All magnesium-based BRS were implanted according to the 4P protocol (patient selection, proper sizing, pre-dilatation, and post-dilatation) and intravascular ultrasound guidance was used in all PCI cases.
The primary outcomes were cardiac death, target vessel myocardial infarction, target lesion revascularisation, and scaffold thrombosis at any time during the follow-up period. Clinical follow-up was scheduled at 3, 6, and 12 months post-PCI. All patients underwent angiographic follow-up at 12 months.
The authors included 37 patients and implanted 57 magnesium-based BRS from February 2017 to September 2018. Mean age was 61 years (standard deviation [SD]: ±10.2 years) and 16.2% of the study cohort presented with myocardial infarction. Mean lesion length was 18.6 mm (SD: ±4.8 mm), 16 lesions (28.0%) were longer than 20 mm, and most lesions were classified as American College of Cardiology/American Heart Association (ACC/AHA) classification B2/C (85.7%). Table 1 shows the incidence of primary outcomes.
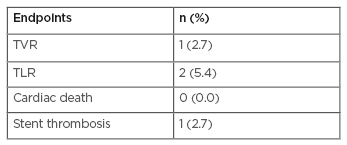
Table 1: Occurrence of primary endpoints in patients treated with magnesium-based bioresorbable vascular scaffolds.
TLR: target lesion revascularisation; TVR: target vessel revascularisation.
All patients received dual antiplatelet treatment with aspirin and clopidogrel during the 12-month follow-up and were instructed to continue dual antiplatelet therapy for 3 years.
CONCLUSIONS
Magnesium-based BRS implantation is feasible and applicable in complex lesions with good acute and mid-term clinical outcomes. Further investigation is needed with a larger number of patients and longer follow-up.








