Meeting Summary
Clinic capacity constraints are an ever-increasing problem in ophthalmology. Multiple case studies demonstrate that faricimab frees up clinic capacity with extended treatment intervals in both treatment-naïve and treatment-experienced patients. In this symposium, three case studies from the UK and Spain demonstrated how fewer appointments per patient with faricimab resulted in several benefits, including timely treatment, reduced treatment burden for patients and caregivers, reduced frequency and cost of out-of-hours services, and freed up clinic staff to manage waiting lists in other ophthalmology services. Ultimately, these outcomes highlight that the introduction of faricimab is cost-effective, leading to better quality of care, the potential for better patient adherence, and less overtime and burnout for clinic staff.
The Clinic Capacity Crisis
In many countries globally, ophthalmology clinics are over capacity, resulting in treatment delays and a reduction in the quality of patient care.1
With rapidly ageing populations, the explosive global increase in diabetes cases, and the rising demand for treatments, this crisis is set to worsen.1-3 In an effort to address this problem, many clinics have implemented solutions such as one-stop clinics1,4 or the use of AI to analyse optical coherence tomography images,1,5 but capacity is still an issue. Increasing access to more durable, innovative therapies may be a sustainable, cost-effective solution to free up capacity, positively impacting patients, caregivers, clinic staff, and payors.1
Faricimab: The First Intraocular Bispecific Antibody
David Wong
Anti-vascular endothelial growth factor (anti-VEGF) monotherapies have been transformative in improving or stabilising vision in patients with neovascular age-related macular degeneration (nAMD), diabetic macular edema (DME), or retinal vein occlusion (RVO).6,7 However, certain patients may fail to respond to treatment, or their response may decrease over time.8-10 These therapies also require frequent intravitreal injections and follow-up visits, creating a strain on clinic capacity11,12 and posing a significant treatment burden on patients and caregivers, which can lead to failure to attend clinics on time and potential subsequent vision loss.12-14
In particular, people with diabetes often have significant co-morbidities that require monitoring, and frequent injections add to this burden.15
Faricimab is the first and only bispecific intraocular antibody that binds and neutralises both angiopoietin-2 and VEGF-A.16,17 Targeting multiple signalling pathways with faricimab may result in durable efficacy when treating retinal diseases.16,17 Clinical trials demonstrated early and sustained visual gains with a reduction in retinal fluid, strong durability with extended treatment intervals, and a favourable benefit-risk profile, with low rates of ocular adverse events.18-25 Faricimab is now approved in more than 100 countries for people living with nAMD, DME, or RVO, with over six million doses distributed globally (as of January 2025).26 The results from clinical trials reflect real-world experience with faricimab;23-25,27-37 an alignment that has not been seen before with anti-VEGF monotherapies.38,39
Real-world evidence has shown that extended treatment intervals with faricimab result in fewer injections and fewer appointments, freeing up capacity with sustained effectiveness and low rates of ocular adverse events.40-44 Ultimately, the introduction of faricimab is an important part of the long-term solution to overcome the clinic capacity crisis.40-44
In this symposium, the speakers discussed how the introduction of faricimab into their practice has freed up capacity, and more specifically how this has impacted patients and caregivers, clinic staff, and hospital managers/payors.
Making a Change for Clinic Staff
Romi Chhabra
The retinal clinic at the Manchester Royal Eye Hospital, UK, sees 180–200 patients per day across four sites, with weekly Saturday clinics to meet the high demand for injections. Saturday clinics are costly and negatively impact the wellbeing of the clinic staff due to overworking and burnout.
Methods
A prospective audit of the real-world effectiveness and safety of faricimab was conducted at the Manchester Royal Eye Hospital in patients starting treatment between 28th November 2022–7th February 2023 (data cut-off 12th January 2024). The audit included 158 treatment-experienced patients with nAMD (200 eyes) who had suboptimal response to prior anti-VEGF monotherapy or disease recurrence upon extension of treatment intervals. Patients met the National Institute for Health and Care Excellence (NICE) reimbursement criteria for anti-VEGF treatments.45 Patients with prior ≤every 4–7 weeks (≤Q4–7W) dosing were initiated on four loading doses of faricimab, while patients with prior ≥Q8W dosing received a matched treatment interval with faricimab. The treatment interval was extended by 2 weeks if the patient demonstrated no disease activity.
The included patients had been treated for a mean of 49.0 months (interquartile range: 19–72 months) prior to switching to faricimab, with a mean (± standard deviation) treatment interval of 5.20 (1.33) weeks in the last 12 months prior to switching. Most patients (88%) were switched from aflibercept to faricimab. The mean (range) length of follow-up after switching was 50.28 weeks (36.9–56.7 weeks).
An additional prospective audit was conducted between 28th November 2022–30th November 2023, which included 129 treatment-naïve patients with nAMD (144 eyes) who were treated with faricimab.
The data from these audits have been provided by Chhabra R (unpublished data).
Results
Treatment-experienced patients demonstrated anatomical improvements and extended treatment intervals:
- After 12 weeks of faricimab treatment, 56% of eyes had an absence of fluid (intraretinal and subretinal) versus 16% of patients pre-switch.
- After 12 months of faricimab treatment, the mean treatment interval was extended by 3 weeks from 5.20 to 8.06 weeks, demonstrating a 55% increase in durability.
- Pre-switch, 0.5% of patients had a ≥Q10W treatment interval; after 12 months of faricimab treatment, this increased to 29.5% of patients (11.5% had a ≥Q14W treatment interval).
- Ocular adverse events were consistent with those seen with anti-VEGF monotherapies, and no new safety signals were noted.
Treatment-naïve patients also demonstrated anatomical improvements and extended treatment intervals after 12 months of faricimab treatment:
- After 12 months, 67% of eyes (N=97) had an absence of fluid (intraretinal and subretinal), versus 0% at baseline.
- After 12 months, 67% of patients had a ≥Q10W treatment interval (26% had a ≥Q14W treatment interval).
- These results align with data reported in the TENAYA/LUCERNE clinical trials.20,21,24,27
Impact on Clinic Staff
Based on the average treatment interval prior to switching compared to 12 months after switching, faricimab reduced the average number of injections by 3.5 per patient per year (from 10 to 6.5). For the 200 eyes included in the audit, this equates to approximately 700 fewer injections per year. Extrapolating to the 700 switch eyes seen at the Manchester Royal Eye Hospital, the introduction of faricimab resulted in approximately 2,450 fewer injections per year.
This freeing up of capacity has decreased the number of Saturday clinics by 75%, from approximately three-to-four clinics per month to one per month, reducing the cost of out-of-hours services. The reduction in the number of appointments at peripheral clinics has allowed for the reallocation of staff to help address waiting lists for other retina clinics (e.g., laser clinics, virtual medical retina reviews), thereby improving the overall efficiency and productivity of the clinic. The reduction in the number of appointments also contributes to a decrease in the carbon footprint associated with clinic visits. Faricimab has reduced capacity strain, which can decrease the feeling of burnout and improve staff wellbeing (Figure 1).
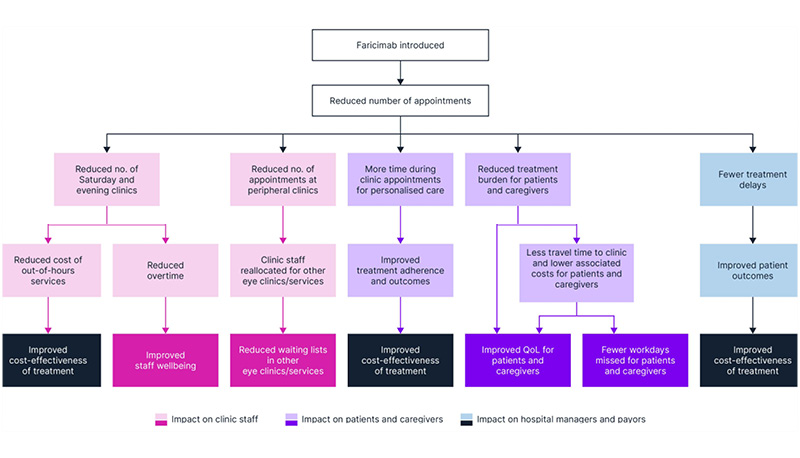
Figure 1: The benefits of faricimab for clinic staff, patients, and caregivers.
no: number; QoL: quality of life.
Faricimab reduced the overall number of injections for patients with nAMD, thereby reducing the frequency of Saturday clinics and associated costs, and improvingclinic resourcing.
Making a Change for Patients
Jorge Ruiz-Medrano
The retinal clinic at the University Hospital Puerta de Hierro Majadahonda, Spain, sees 28–30 patients per day. Prior to the introduction of faricimab, patients were experiencing multiple challenges related to capacity, including long waiting times, delayed appointments, and limited interaction with healthcare professionals during their appointment. Importantly, the treatment outcomes were also occasionally falling short of expectations due to delayed appointments and poor patient adherence.
Methods
A prospective audit of the real-world effectiveness and safety of faricimab was initiated on 1st October 2023. The audit included 245 treatment-experienced patients with nAMD on ≤Q10W for at least 1 year. Patients with additional ocular diseases other than cataracts were excluded. Patients started at their prior dosing schedule, with their treatment interval extended at 4-week increments upon disease control (including no/minimal retinal fluid present). The interim data reported in this symposium had a cut-off date of 1st September 2024; the final data cut-off date for this audit was the 31st December 2024.
The included patients had a mean (± standard deviation) treatment interval of 6 (1.97) weeks prior to switching to faricimab. Most patients (65.6%) were switched from aflibercept to faricimab.
The data from this audit have been provided by Ruiz-Medrano J (unpublished data).
Results
After four faricimab injections, treatment intervals were extended (N=146):
- The mean treatment interval was extended by 4 weeks from 5.5 to 9.6 weeks, demonstrating a 75% increase in durability.
- Pre-switch, 6% of patients had a ≥Q10W treatment interval; after four faricimab injections, this increased to 49% of patients (17% had a ≥Q14W treatment interval).
- Faricimab was well tolerated; the incidence of adverse events was consistent with that seen in clinical trials.19,20
Impact on Patients and Caregivers
Based on the average treatment interval prior to switching compared to after four faricimab injections, faricimab reduced the average number of injections by 3.5 per patient per year (from 8.7 to 5.2). For the 146 patients included in the audit, this equates to approximately 506 fewer injections per year. Extrapolating to the 400 switch patients seen at the University Hospital Puerta de Hierro Majadahonda, the introduction of faricimab would result in approximately 1,400 fewer injections per year, translating to a 20% reduction in the number of appointments.
Since the introduction of faricimab, clinicians have reported more time during clinic appointments for personalised care and have used this time to show patients their optical coherence tomography images, explain the outcomes in more detail, and have longer discussions regarding any concerns. This additional time may contribute to improved adherence and patient outcomes.
Patients and caregivers could also benefit from an improved quality of life, with reduced treatment burden and less time needed to travel to a clinic. These improvements are particularly relevant for patients with DME of working age, who would experience fewer missed workdays associated with appointments (Figure 1).
Faricimab reduced the number of appointments, allowing physicians to spend more time with each patient and improving quality of life for patients and their caregivers.
Making a Change for Hospital Managers and Payors
Robin Hamilton
The retinal clinic at Moorfields Eye Hospital, UK, administers approximately 5,000 injections per month, with injection sites across various centres and costly Saturday clinics to manage overflow. To manage budgets, there is increasing pressure to use cost-saving measures such as prescribing more biosimilars.
Methods
A prospective audit of the real-world effectiveness and safety of faricimab was conducted at Moorfields Eye Hospital between 20th September 2022–13th March 2024.30,46 The audit included 172 treatment-naïve patients with nAMD who met the NICE reimbursement criteria for anti-VEGF treatments.45 Patients were initiated onto faricimab with four loading doses. The treatment interval was extended in patients with a good response (defined as no intraretinal fluid, subretinal fluid, or subretinal haemorrhage, and stable pigment epithelium detachment).
Results
After 12 months of faricimab treatment, treatment-naïve patients demonstrated anatomical improvements and extended treatment intervals:
- After 12 months, 68.9% of eyes (N=125) had an absence of fluid versus 0% at baseline.
- After 12 months, 74.6% of patients had a ≥Q10W treatment interval (38.6% had a ≥Q14W treatment interval), with a mean treatment interval of 11.4±3.43 weeks (median 12 weeks).
- These results align with data reported in the TENAYA/LUCERNE clinical trials.20,21,24,27
- Faricimab was well tolerated; the incidence of adverse events was consistent with that seen in clinical trials.47
The outcomes in treatment-experienced patients at Moorfields Eye Hospital reflect those seen at the Manchester Royal Eye Hospital and University Hospital Puerta de Hierro Majadahonda:46
- Treatment intervals were extended by a mean of 3 weeks over 12 months, from 4 weeks to 7 weeks.
Impact on Hospital Managers and Payors
With the rising pressure to use cheaper, less durable treatment options such as biosimilars, clinic data can be leveraged to demonstrate the benefits of, and increase access to, durable therapies like faricimab.48,49
Demonstrate the impact of the rising demand for intravitreal injections on future clinic capacity
Demand for intravitreal injections at Moorfields Eye Hospital is expected to increase by 1.8-fold over 10 years, from 44,924 injections per year in 2019 to82,867 in 2029.50
Build a strong cost-benefit narrative that considers more than the cost of the treatment
Cost-benefit narratives for durable therapies should include the cost of treatment, treatment outcomes, predicted annual number of injections, costs of delivering treatment (including infrastructure and staff costs), costs experienced by patients and caregivers, and overall costs associated with vision loss (Figure 2).
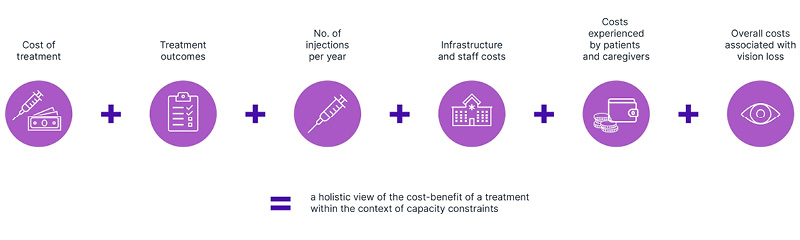
Figure 2: Building a comprehensive cost-benefit narrative, considering more than the cost of treatment.
These factors should be considered within the context of clinic capacity. For example, in clinics with capacity constraints, treatment outcomes may be suboptimal due to treatment delays, and costs of delivering treatment are likely to increase due to the need for out-of-hours services (e.g., Saturday clinics).11,51-53 To avoid the negative impact of capacity strain, one option is to build more infrastructure and/or employ more staff; this option is associated with significant costs and will take time to come into effect.1,50,54 Another, and more cost-effective, option is to adopt durable therapies within existing capacity levels.1,50
Collect clinic data to demonstrate patient outcomes and cost-benefit of the treatment
To demonstrate the cost-benefit of faricimab, a patient-level simulation model was conducted with a 5-year time horizon, comparing faricimab with aflibercept 2 mg and with a ranibizumab biosimilar.48 The model assumed 1,500 patients with nAMD and 500 patients with DME, treated at a typical UK eye hospital with 17 4-hour injection sessions per week.
Treatment with biosimilars was predicted to consistently exceed clinic capacity by approximately 3,000–4,000 appointments per year, while the number of appointments associated with faricimab was predicted to be consistently below the clinic capacity limit, meaning that capacity is freed up. The capacity strain associated with biosimilars may result in treatment delays leading to unnecessary sight loss and out-of-hours services, while with faricimab, patients can be treated on time, leading to improved treatment outcomes.
Data from Moorfields Eye Hospital validate the simulation model, showing that since the introduction of faricimab into clinical practice, the overall number of intravitreal injections is stabilising, further demonstrating the positive impact of faricimab on capacity (Hamilton R, unpublished data) (Figure 3).
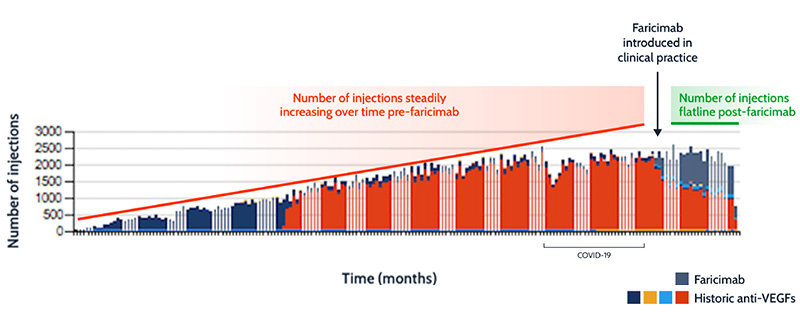
Figure 3: The impact of faricimab on overall number of injections at Moorfields Eye Hospital.
Trend lines are illustrative.
VEGF: vascular endothelial growth factor.
Engage with key stakeholders
Engaging with payors, hospital managers, and pharmacists is essential to enable access to durable therapies. When communicating with these stakeholders, emphasise the importance of timely treatment and a reduced treatment frequency to achieve optimal outcomes.
Adoption of faricimab in clinical practice has freed up clinic capacity, allowing optimal patient outcomes and demonstrating cost-effectiveness for hospital managers and payors (Figure 1).
Increasing Access to Durable Therapies
The real-world experience demonstrated by the speakers’ case studies, as well as the broader real-world experience with faricimab,35-37,40-44 show that this treatment is durable and efficacious, with a favourable benefit-risk profile,47 and provides benefit to all stakeholders, including clinic staff, patients and caregivers, and hospital managers and payors.
How Can Other Healthcare Professionals Leverage These Data and Learnings in Their Own Clinical Practice?
- Identify the key stakeholders to engage with, including payors, hospital managers, and pharmacists.
- Emphasise that the current clinic capacity crisis will worsen with the rising demand for intravitreal injections.50 A long-term, cost-efficient solution is needed to free up capacity without compromising on treatment outcomes.
- Collect clinic data and leverage these to build a strong cost-benefit narrative (Figure 2). Published economic analyses on the impact of durable therapies can provide a framework when developing a similar analysis in your practice.11,48,55
- Advocate for the first-line use of durable therapies such as faricimab over the use of cheaper, less durable therapies such as biosimilars, based on the positive treatment outcomes, freeing up of capacity which allows waiting lists for other ophthalmology services to be reduced, and other associated cost savings.48,49
Freeing up clinic capacity will improve clinic experience, reduce treatment burden, and improve quality of life for patients and caregivers. For these reasons, adopting innovative and durable therapies into clinical practice is a change for good.







