Abstract
This review describes the evidence that forms the foundation for the use of an implantable cardiac defibrillator (ICD). The authors present the current guidelines for the implantation of ICDs and describe the most important historic and latest clinical trials that have been conducted to examine the role of ICDs for primary and secondary prevention of a cardiac arrest. Finally, the authors discuss new technologies that have been developed to improve outcomes and reduce adverse events associated with these types of cardiac implantable electronic devices.
INTRODUCTION
Since its first clinical use in 1980,1 implantable cardiac defibrillators (ICD) have become a standard tool in the treatment of heart failure (HF) and prevention of sudden cardiac death (SCD) in patients fortunate enough to have survived a cardiac arrest. Cardiologists are commonly faced with the decision of whether to refer patients for ICD implantation based on the individual patient characteristics and best available evidence. This review aims to reflect on the evolving evidence about ICDs from pivotal clinical trials that have shaped and expanded its indication in modern cardiology. The authors will also explore the emerging role of subcutaneous ICD (S-ICD) as an alternative to the conventional ICD device.
Current Guideline Recommendation
The latest European Society of Cardiology (ESC) guidelines on HF promote the implantation of ICD not only in patients who survived a cardiac arrest caused by a ventricular arrhythmia (i.e., secondary prevention), but also in patients classified as at high risk of SCD (i.e., primary prevention). Besides channelopathies and congenital heart disease, primary prevention primarily involves patients with significant left ventricular systolic dysfunction, left ventricular ejection fraction (LVEF) ≤35% due to ischaemic (ICM) or non-ischaemic cardiomyopathy (NICM) with New York Heart Association (NYHA) Class II–III symptoms despite optimal medical therapy (OMT) for >3 months and a life expectancy of >1 year.2
Historical Evidence for Secondary Prevention
The guidance on secondary prevention was instigated by the historic AVID3 and CASH4 trials, which began recruitment in the late 1980s. Although these trials were conducted over two decades ago when HF treatments were limited to ß-blockers and angiotensin-converter enzyme inhibitors, they provided a strong foundation for the prognostic benefits of ICD in patients with unstable ventricular arrhythmia or survivors of cardiac arrest. CASH and AVID found a 20% and 39% relative risk reduction of mortality, respectively.3,4 At the beginning of CASH, ICDs were still epicardial systems which were implanted by cardiothoracic surgeons via thoracotomy. Over the course of the trial, miniaturisation and development of endocardial leads revolutionised the approach and implantation of transvenous ICD (TV-ICD) as we know today.
Primary Prevention Implantable Cardioverter Defibrillator for Ischaemic Cardiomyopathy
The basis for the recommendation of preventative ICD for patients who have never experienced a life-threatening arrhythmia or cardiac arrest is more elaborate. Initial trials were largely based on patients considered at high risk for life-threatening arrhythmias. Published in 1996, MADIT 1 recruited 196 patients with a previous myocardial infarction (MI) and LVEF ≤35%. Compared to the OMT cohort, there was a 54% reduction of all-cause mortality in the defibrillator-treated group (p=0.009). However, this study population not only had to have documented evidence of previous ventricular tachycardia (VT) but also had to undergo an electrophysiology study to elicit VT.5 The study findings were corroborated in the larger MUSTT trial.6 Similarly, patients were selected on the basis of an episode of asymptomatic non-sustained VT at least 4 days post-MI with evidence of inducible VT on electrophysiology study in the intervention arm, but with a slightly less stringent LVEF of ≤40%. In a three-arm design, it found that electrophysiology (EP)-guided therapy with a defibrillator resulted in a 50% reduction in total mortality and a 70% reduction in arrhythmic death, compared to patients assigned to either EP-guided medical therapy or no antiarrhythmic therapy.6
The MADIT II trial, published in 2002, took a practical stance and obviated the need for EP testing before enrolment.7 In a similar population of patients post-MI with systolic dysfunction (LVEF: ≤30%), prophylactic ICD was found to improve survival, with a 31% relative risk reduction in all-cause mortality compared to medical therapy only (hazard ratio [HR]: 0.69, p=0.016). However, like the preceding studies, it excluded patients with recent MIs within the last month. To address this gap, DINAMIT, published 2 years later, enrolled patients with an MI between 6 and 40 days earlier, LVEF ≤35%, and impaired cardiac autonomic function.8 No clinical benefit was found in patients with a recent MI; this was subsequently reflected in the ESC guidelines.²
TRANSLATION OF EVIDENCE TO NON-ISCHAEMIC CARDIOMYOPATHY
While evidence of ICD for ICM accumulated, investigators recognised the need to explore the same for patients with NICM. The SCD-HeFT trial was sufficiently powered to examine this.9 Over 2,500 patients with severe left ventricular systolic dysfunction and an equal distribution of ICM and NICM were randomised to either ICD, amiodarone, or placebo. At 4 years follow-up, ICD therapy significantly reduced all-cause mortality for both NICM and ICM compared to the other arms (HR: 0.77, p=0.007). That said, in a subgroup analysis of NICM alone (n=792), only a non-significant trend towards reduced mortality was found (HR: 0.73; 97.5% confidence interval [CI]: 0.50–1.07).9
A comparable study population of patients with ICM and NICM was recruited in the COMPANION trial but for the first time, patients had to have a QRS prolongation (>120 msec) to study the effects of cardiac resynchronisation therapy (CRT) on a composite primary endpoint of all-cause mortality and hospitalisation. Randomised to three arms of OMT, OMT plus biventricular pacing, and OMT plus biventricular defibrillator (CRT-D), the study was halted early due to demonstration of superior efficacy in both resynchronisation arms on its primary outcome (HR: 0.81, p=0.015). Furthermore, it was noted that CRT-D had a greater effect on all-cause mortality than CRT alone, galvanising studies to explore this further.10
Cardiac Resynchronisation Therapy
Is the degree of QRS prolongation a target to improve mortality? The MADIT-CRT trial began recruitment in 2004:11 1,820 patients with NICM and ICM, LVEF ≤30% but with milder HF symptoms (NYHA I–II), and a QRS duration ≥130 msec were randomised to receive either a biventricular defibrillator or an ICD alone. Primary outcome was death from any cause or non-fatal HF events. At 2.5 years follow-up, there was a 30% relative risk reduction in the primary endpoint in the biventricular defibrillator group compared to ICD alone (HR: 0.66; 95% CI: 0.52–0.84; P=0.001).11 This provided convincing evidence that preventive CRT-D reduces HF events in patients with either ICM or NICM with a wide QRS complex and relatively well-controlled HF symptoms. In a subgroup analysis of patients with left bundle branch block (LBBB), the primary outcome was even more pronounced (HR: 0.43; 95% CI: 0.33–0.56; p<0.001) while non-LBBB demonstrated less improvement of LVEF with no significant reduction in SCD.12 Based on MADIT-CRT and COMPANION, it is now a Class I recommendation to offer a biventricular defibrillator rather than conventional ICD to patients with chronic HF, LVEF ≤35%, and at least NYHA Class II with LBBB and QRS duration ≥130 msec.2
While the Echo-CRT trial was stopped early for futility and possible harm of CRT in patients with HF with reduced ejection fraction (HFrEF) with narrow QRS complexes (<130 msec),13 there are specific patient populations that may prognostically benefit from CRT despite a narrow QRS duration. Apart from patients with HFrEF who require chronic right ventricular pacing for bradyarrhythmia,14 one such population was highlighted in the recent APAF-CRT trial, which was stopped early for efficacy of its ‘ablate and pace’ strategy in narrow-QRS HF.15 In this open-label trial, 133 patients (mean age: 73±10 years) with severely symptomatic permanent atrial fibrillation and at least one HF hospitalisation in the last year were randomly assigned to either atrioventricular junction ablation plus CRT or pharmacological rate control. The primary endpoint was all-cause mortality. After a median 29 months follow-up, the former intervention was associated with a substantial 74% reduction in death (HR: 0.26; 95% CI: 0.10–0.65; p=0.004) with no significant differences in benefit between patients with LVEF below or above 35%. The number needed to treat to prevent one event was 3.7.15 From this, there is a suggestion of additional benefit in CRT for patients who are candidates for atrioventricular junction ablation to optimise atrial fibrillation rate control in patients with HF and narrow QRS. Nonetheless, a large sample size is required before confidently adopting this approach.
The Role of Implantable Cardioverter Defibrillators in a Modern Era of Guideline-Directed Therapies
Guideline-directed medical therapies for HFrEF have progressed. The arrival of sacubitril/valsartan and sodium-glucose cotransporter-2 inhibitors (SGLT2i) have demonstrated strong mortality benefits in this patient group.16,17 With this in mind, will the efficacy of ICD in preventing SCD remain despite a greater armamentarium of HF drugs? Trials comparing biventricular defibrillators versus biventricular pacemakers in patients eligible for primary prevention ICD will hopefully shed light on this.18
Partially addressing this question for the NICM population is the DANISH trial, published 5 years ago. Recognising the gradual reduction of SCD rates over the last 20 years with greater uptake of HF pharmacotherapies, it recruited the largest population of patients with NICM (N=556) with LVEF ≤35% to examine the mortality benefits of ICD. Over 67.6 months, all-cause mortality was no different between the ICD versus control group (HR: 0.87; 95% CI: 0.68–1.12; p=0.28). More importantly, its overall rate of SCD was much lower than previous trials, and this trend is likely to continue since the advent of sacubitril/valsartan and SGLT2i, which were unavailable during DANISH.19
As summarised in Table 1 of the above clinical trials, it took the last 25 years to collect evidence that patients with ICM and LVEF ≤35% benefit from ICD implantation, and do even better when treated with a biventricular defibrillator if the QRS duration is prolonged. On the other hand, patients with NICM seem not to benefit from a defibrillator on the whole.
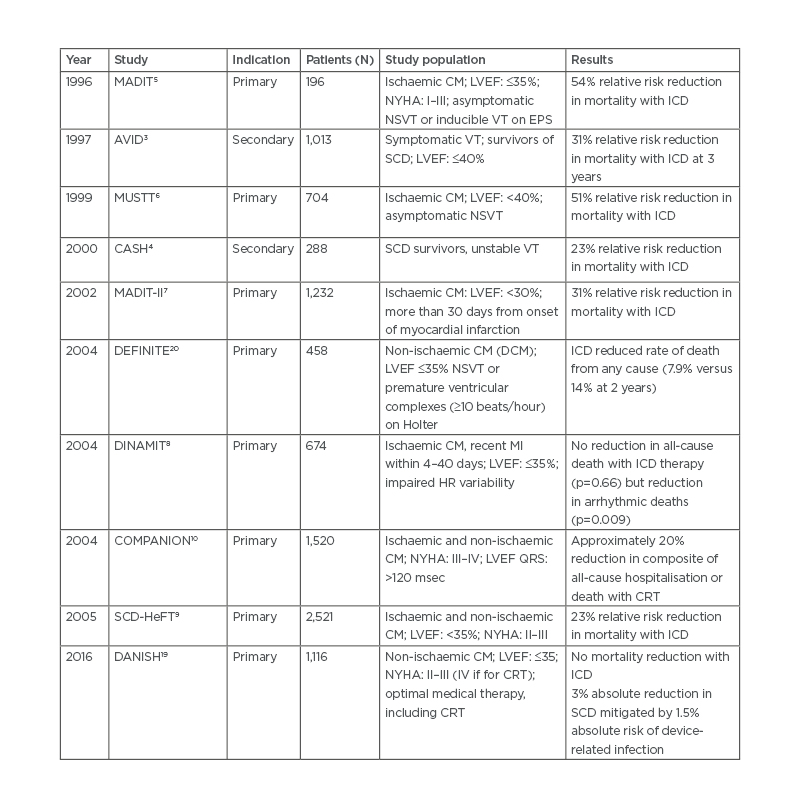
Table 1: Summary of major clinical trials of implantable cardioverter defibrillators.
CM: cardiomyopathy; CRT: cardiac resynchronisation therapy; DCM: dilated cardiomyopathy; EPS: electrophysiology study; HR: heart rate; ICD: implantable cardioverter defibrillator; LVEF: left ventricular ejection fraction; NSVT: non-sustained ventricular tachycardia; NYHA: New York Heart Association; SCD: sudden cardiac death; VT: ventricular tachycardia.
Subcutaneous Versus Transvenous Implantable Cardioverter Defibrillator
Another point highlighted by DANISH was the substantial complication rate in the ICD group, offsetting the 3% reduction in mortality; 3% of patients had a serious device-related infection, 2% experienced pneumothorax, and 6% received inappropriate shocks (IAS).19 The inherent risks associated with the implantation of TV-ICDs may be circumvented by the pioneering technology of subcutaneously implanted defibrillators. As illustrated in Figure 1, the S-ICD lead is tunnelled subcutaneously on top of the rib cage cranially towards the suprasternal notch. The distal sensing electrode is placed adjacent to the manubriosternal notch and the proximal sensing electrode near the xiphoid process. The extra-thoracic device lies on the left thoracic region, close enough to the heart to sense the far-field ECG and terminate any life-threatening ventricular arrhythmia.21
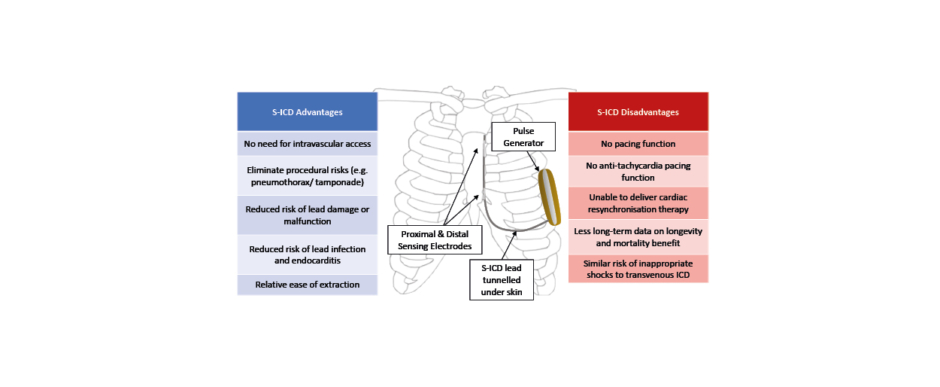
Figure 1: Position of subcutaneous ICD and its advantages and disadvantages.
ICD: implantable cardioverter defibrillator; S-ICD: subcutaneous implantable cardioverter defibrillator.
Based on the pros and cons of S-ICD (Figure 1), it is usually recommended in younger patients, those with a history of vascular access problems, or those considered to be at high risk for device-related infections. By virtue of its design, S-ICD would not be appropriate in patients who require bradycardia pacing, CRT, or a need for anti-tachycardia pacing. Furthermore, due to its reliance on far-field sensing as opposed to near-field electrograms detected by TV-ICD, the risk of IAS from cardiac oversensing was relatively higher.22,23 Software and hardware modifications over the last 10 years have improved this. One key development is the SMART-PASS filtering algorithm, which lowers the T-wave amplitude in order to minimise the risk of IAS from T-wave oversensing.24
The potential to combine S-ICD with a leadless pacemaker is another attractive solution to utilise the benefits of both systems of a less invasive defibrillator while providing anti-tachycardia pacing delivery and pacing.
Although S-ICD has been approved for use in Europe over the last 10 years,21 its evidence base is still in its infancy compared with TV-ICD. PRAETORIAN is the first randomised trial comparing subcutaneous versus transvenous defibrillators.25 The trial was designed and powered as a non-inferiority trial with a safety primary composite endpoint of device-related complications and IAS.
Researchers recruited 849 patients who had an indication for ICD but not for pacing. At a median follow-up of 49.1 months, there was no significant difference in primary outcome (HR: 0.99; 95% CI: 0.71–1.39; p=0.01 for non-inferiority). This suggested that in the absence of the need for pacing or resynchronisation, S-ICD appeared non-inferior to TV-ICD. It is important to note that the high-pass filter function for S-ICD was unavailable in >75% of cases since PRAETORIAN began, and hence the risk of IAS may be lower than observed.25
Hopefully, a clearer picture of the differences between S-ICD and TV-ICD will be known as the trial continues for a further 4 years.
REDUCING THE RISK OF INAPPROPRIATE SHOCKS FROM SUBCUTANEOUS IMPLANTABLE CARDIOVERTER DEFIBRILLATORS
Even now, we can already see how far technology has progressed with S-ICDs in reducing IAS. From the first large real-world data on S-ICD in EFFORTLESS,22,23 a high IAS rate of 8.1% was reported. This is lower than that observed with earlier TV-ICD in the era of MADIT-II and SCD-HeFT where an IAS rate between 13% and 17% was described.7,9 Obviously, device programming has advanced since then. PRAETORIAN revealed an IAS rate of 4.1% with TV-ICD versus 4.9% for S-ICD.25 An even lower rate of 2.5% was found in the recent UNTOUCHED study, which employed contemporary electrogram filtering and algorithms.26 These modern devices not only had SMART-PASS filtering but were also programmed with a conditional zone of 200 bpm (using beat-to-beat and QRS width analyses) to differentiate supraventricular from ventricular arrhythmias and shock zone of 250 bpm.26
Clinical Implication
This review highlights the constantly shifting landscape of ICD implantation, dictated by its dynamic and developing evidence base. Emphasised in the latest 2021 ESC guidelines for the management of chronic HF27 and use of CRT,14 an important clinical implication is the careful selection of patients and multi-disciplinary involvement before pursuing primary prevention ICD implantation. Recommendation for primary preventative ICD in the NICM population (based on the same indications as ICM) has been downgraded to Class IIa in light of the DANISH trial and a meta-analysis.19,27,28 The falling burden of SCD and cardiovascular mortality in the NICM population attributed to the use of contemporary HF therapy makes it difficult for ICDs to further reduce the risk of SCD. That said, with careful patient selection, young patients with little comorbidity may still benefit. Another area where careful patient selection is key is the use of S-ICD. When used in appropriately selected patient groups, it can be used as a non-inferior alternative in safety and efficacy to TV-ICDs.14,27
CONCLUSION
We have come a long way since the first clinical use of ICD in the early 1980s. The advances of ICD technology have made it safer and effective to prevent SCD and treat HF in both ICM and NICM. It has resulted in a striking global increase in the implantation of defibrillators and resynchronisation devices with its indications continually being redefined and expanded by ongoing research. S-ICDs have provided a valuable alternative to the transvenous type, which is implanted on an individualistic approach and continues to evolve to overcome its current shortcomings.








